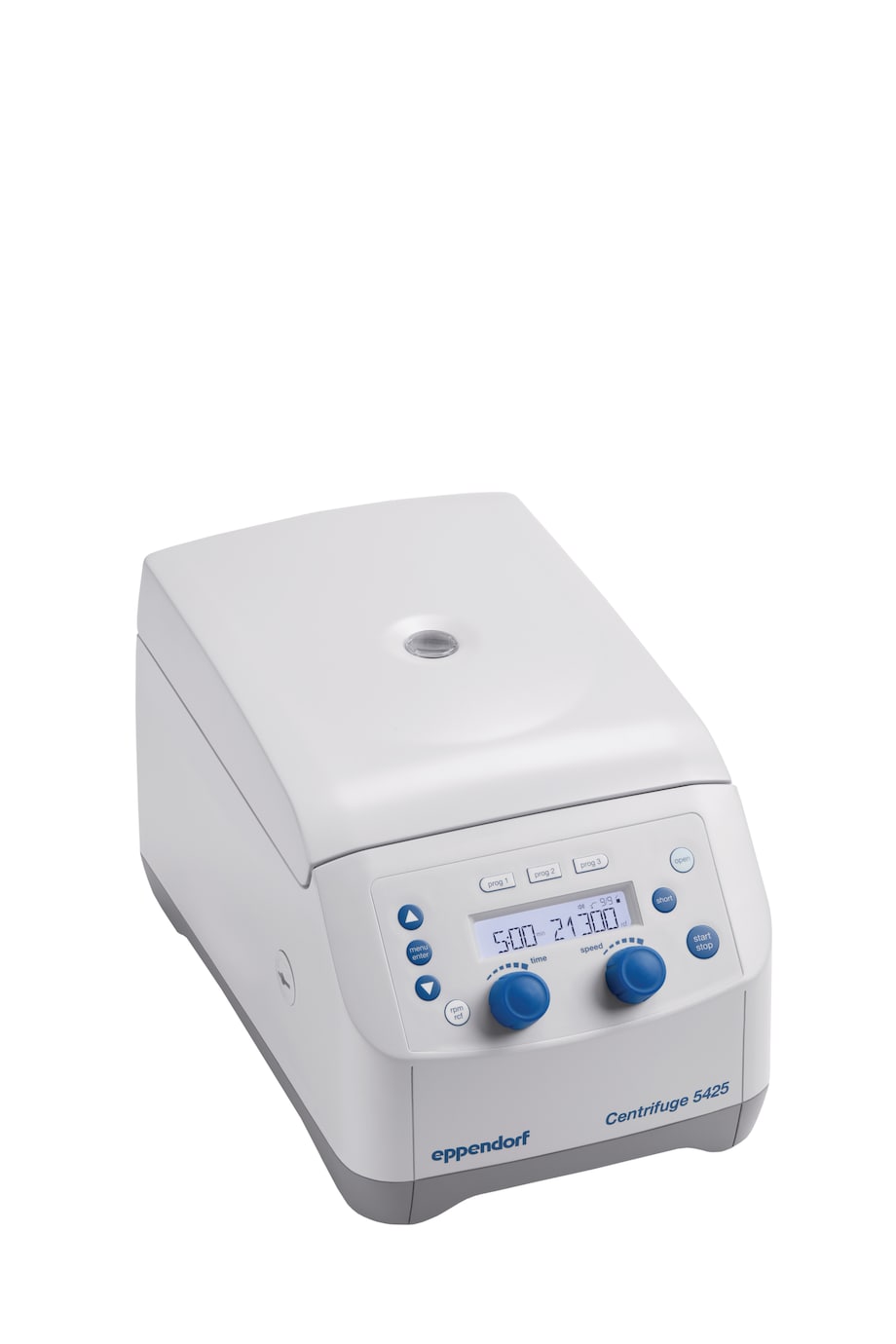
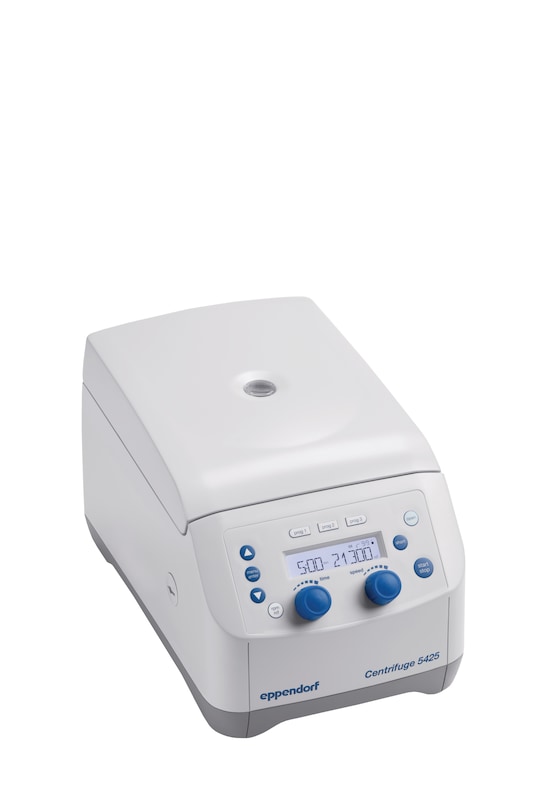
- with rotary knob control panel for quick parameter changes
- without rotor
- non-refrigerated
- 230 V, 50 – 60 Hz
MENU
ID | IDR
ID | IDR
-
- All Centrifuges
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- IVD Products
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Pipette Tips
- Bottle-Top Dispensers
- Pipette Controllers
- Dispenser & Pipette Accessories
- Automated Pipetting
- Automation Consumables
- Automation Accessories
- Liquid Handler & Pipette Services
Sorry, we couldn't find anything on our website containing your search term.
You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.
Sorry, we couldn't find anything on our website containing your search term.
- Home
- Products
- Centrifugation
- IVD Products
- Centrifuge 5425/5425 R
- 5405000611
Centrifuge 5425 (IVD)

Medical Device registration in Indonesia: Click here for Kementerian Kesehatan Republik Indonesia product listing
All medical devices sold in Malaysia must carry local labelling as per requirement from the Medical Device Authority. To allow this to happen, the packaging of the non-refrigerated devices will be opened and resealed for labelling purposes.
Medical Device registration in Indonesia: Click here for Kementerian Kesehatan Republik Indonesia product listing
All medical devices sold in Malaysia must carry local labelling as per requirement from the Medical Device Authority. To allow this to happen, the packaging of the non-refrigerated devices will be opened and resealed for labelling purposes.
All medical devices sold in Malaysia must carry local labelling as per requirement from the Medical Device Authority. To allow this to happen, the packaging of the non-refrigerated devices will be opened and resealed for labelling purposes.
-
IVD
Product Information
- rotary knobs, non-refrigerated
- without rotor
- 230 V, 50 – 60 Hz, EU

-
Request a demo
You will find additional download material at the bottom of this page
Features