メニュー
JP | JPY
-
-
-
- Challenges and Chances: A Review of the 1st Stem Cell Community Day
- Summertime, and the Livin’ Is Easy…
- Follow-on-Biologics – More than Simple Generics
- Bacteria Versus Body Cells: A 1:1 Tie
- Behind the Crime Scene: How Biological Traces Can Help to Convict Offenders
- Every 3 Seconds Someone in the World Is Affected by Alzheimer's
- HIV – It’s Still Not Under Control…
- How Many Will Be Convicted This Time?
- Malaria – the Battle is Not Lost
- Physicians on Standby: The Annual Flu Season Can Be Serious
- At the Forefront in Fighting Cancer
- Molecular Motors: Think Small and yet Smaller Again…
- Liquid Biopsy: Novel Methods May Ease Cancer Detection and Therapy
- They Are Invisible, Sneaky and Disgusting – But Today It’s Their Special Day!
- How Many Cells Are in Your Body? Probably More Than You Think!
- What You Need to Know about Antibiotic Resistance – Findings, Facts and Good Intentions
- Why Do Old Men Have Big Ears?
- The Condemned Live Longer: A Potential Paradigm Shift in Genetics
- From Research to Commerce
- Chronobiology – How the Cold Seasons Influence Our Biorhythms
- Taskforce Microbots: Targeted Treatment from Inside the Body
- Eyes on Cancer Therapy
-
-
-
-
- Challenges and Chances: A Review of the 1st Stem Cell Community Day
- Summertime, and the Livin’ Is Easy…
- Follow-on-Biologics – More than Simple Generics
- Bacteria Versus Body Cells: A 1:1 Tie
- Behind the Crime Scene: How Biological Traces Can Help to Convict Offenders
- Every 3 Seconds Someone in the World Is Affected by Alzheimer's
- HIV – It’s Still Not Under Control…
- How Many Will Be Convicted This Time?
- Malaria – the Battle is Not Lost
- Physicians on Standby: The Annual Flu Season Can Be Serious
- At the Forefront in Fighting Cancer
- Molecular Motors: Think Small and yet Smaller Again…
- Liquid Biopsy: Novel Methods May Ease Cancer Detection and Therapy
- They Are Invisible, Sneaky and Disgusting – But Today It’s Their Special Day!
- How Many Cells Are in Your Body? Probably More Than You Think!
- What You Need to Know about Antibiotic Resistance – Findings, Facts and Good Intentions
- Why Do Old Men Have Big Ears?
- The Condemned Live Longer: A Potential Paradigm Shift in Genetics
- From Research to Commerce
- Chronobiology – How the Cold Seasons Influence Our Biorhythms
- Taskforce Microbots: Targeted Treatment from Inside the Body
- Eyes on Cancer Therapy
-
JP | JPY
How do I Monitor the Growth Rate of My Microbial Culture?
Natascha Weiß Lab Academy
- 微生物学
- 実験室の日常業務
- 微生物の培養
- 測光
- エッセー
Can I proceed with my prokaryotic cells or do I still need to wait another hour? A sufficient growth rate is needed for microbial cells before you start to harvest or to start your main culture. A typical growth check can be done by OD600 with your photometer.
Microorganisms such as bacteria and yeast are employed in a multitude of experiments in microbiology and molecular biology laboratories. In order to propagate them, a liquid medium containing all necessary nutrients is typically inoculated with a colony or a starter culture and subsequently incubated for several hours. The incubation time depends on the organism, the culture conditions and the intended use of the culture.
Many applications require harvesting the cells during the exponential growth phase (log phase) in order to obtain the highest possible number of live cells. The right time for harvest may be based on experience; however, it is safer to monitor culture growth on a regular basis. One quick, simple and inexpensive method is to measure the optical density at 600 nm (OD600).
Molecules such as nucleic acids and proteins, which exist in solution, absorb light at defined wavelengths. A culture of microorganisms, on the other hand, constitutes a suspension, which absorbs very little light during photometric measurements. Instead, cells deflect the light, which is the reason that the OD600 method is also known as scattered light measurement or turbidimetry (figure 1). However, the measuring principle of both methods is the same in the photometer: Light is sent through a sample and the original light intensity is compared with the light intensity attenuated by the sample.
Many applications require harvesting the cells during the exponential growth phase (log phase) in order to obtain the highest possible number of live cells. The right time for harvest may be based on experience; however, it is safer to monitor culture growth on a regular basis. One quick, simple and inexpensive method is to measure the optical density at 600 nm (OD600).
Molecules such as nucleic acids and proteins, which exist in solution, absorb light at defined wavelengths. A culture of microorganisms, on the other hand, constitutes a suspension, which absorbs very little light during photometric measurements. Instead, cells deflect the light, which is the reason that the OD600 method is also known as scattered light measurement or turbidimetry (figure 1). However, the measuring principle of both methods is the same in the photometer: Light is sent through a sample and the original light intensity is compared with the light intensity attenuated by the sample.
もっと読む
表示を減らす
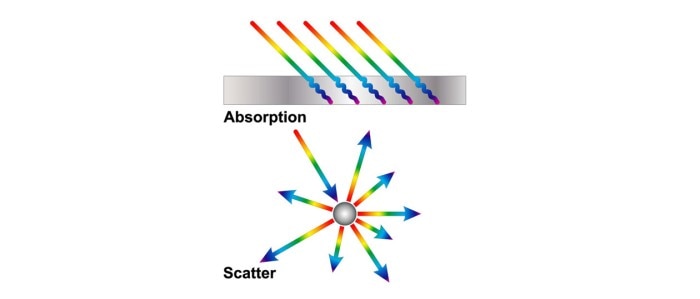
Figure 1: Two possible interactions of light with substances. In the case of absorption, the substance absorbs the light or part of it. Scatter results from the deflection of light in different directions.
Many instruction manuals for the culture of microorganisms in liquid media state the culture is to be harvested at an OD600 value. Such a value, however, is only meaningful in the context of the specific bacterial strain that is cultured under defined growth conditions and measured using a specific instrument.
The reason for such requirements is that, in contrast with absorbance measurements, the light scatter that occurs during turbidity measurements is strongly dependent on the size and shape of the cells as well as the optical design of the photometer [1, 2]. Different strains thus scatter light in different ways, and different growth conditions may also influence the size and the shape of a cell. The influence of the photometer becomes evident once the sample is measured using different instrument types; discrepancies as high as 100% of the measured OD600 values are not unheard of [2]. With respect to the intensity and the angle of incidence of the stray light that reaches the detector of the photometer, the distance between the aperture and the detector, as well as the size of the aperture behind the cuvette shaft, are of critical importance (figure 2) [2].
The reason for such requirements is that, in contrast with absorbance measurements, the light scatter that occurs during turbidity measurements is strongly dependent on the size and shape of the cells as well as the optical design of the photometer [1, 2]. Different strains thus scatter light in different ways, and different growth conditions may also influence the size and the shape of a cell. The influence of the photometer becomes evident once the sample is measured using different instrument types; discrepancies as high as 100% of the measured OD600 values are not unheard of [2]. With respect to the intensity and the angle of incidence of the stray light that reaches the detector of the photometer, the distance between the aperture and the detector, as well as the size of the aperture behind the cuvette shaft, are of critical importance (figure 2) [2].
もっと読む
表示を減らす
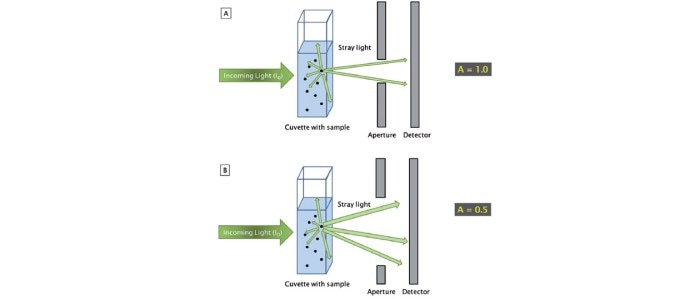
Figure 2: The different optical designs of photometers A and B lead to a change in the amount of light that reaches the detector and, as a consequence, to different results when measuring stray light from identical sample concentrations.
If the measured optical density is to be expressed in relation to a culture parameter such as, for example, the cell number, it is necessary to calibrate the photometer. In order to determine the concentration of the cells, OD600 values are aligned with the number of cells of the cultured strain. Otherwise, there is a possibility that aberrant measurement values may lead to a false interpretation of the growth phase of the culture, and in the worst case, the subsequent experiment may not be successfully carried out.
[1] Janke SA, Fortnagel P, Bergmann R. Microbiological turbidimetry using standard photometers. BIOspektrum, 1999; Vol. 6: 501-502.
[2] Harnack K, Spolaczyk R, Janke SA. Turbidity measurements (OD600) with absorption spectrometers. BIOspektrum, 1999; Vol. 6: 503-504.
References:
[1] Janke SA, Fortnagel P, Bergmann R. Microbiological turbidimetry using standard photometers. BIOspektrum, 1999; Vol. 6: 501-502.[2] Harnack K, Spolaczyk R, Janke SA. Turbidity measurements (OD600) with absorption spectrometers. BIOspektrum, 1999; Vol. 6: 503-504.
もっと読む
表示を減らす

