You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.

Increase the Reproducibility of Your Cell Culture Bioprocess
ULRIKE RASCHE Lab Academy
- Bioprocessing
- Cell Culture
- Reproducibility
- Bioprocess
- BioNews article
Reproducible cell growth and reliable production of the desired product – this is the ideal scenario for any bioprocessing engineer! After all, the poorer the reproducibility, the higher the risk of needing to discard the batch and repeat the entire bioprocess – at a high cost in terms of time, resources, and nerves. We asked the bioprocessing specialists in our Eppendorf Application Laboratory to find out which factors are likely to lead to inconsistent results, and we received valuable tips that can help decrease the batch-to-batch variation of cell culture bioprocesses.
Tip 1: Start with equal conditions
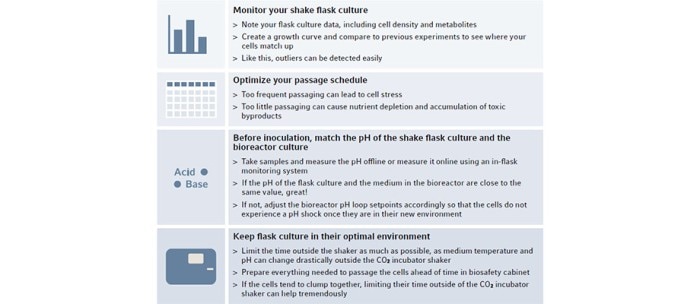
A bioprocess does not begin inside the bioreactor. The cells used for inoculation have a history: typically, they were thawed, expanded in a stepwise manner in shaker flasks and then transferred to the reactor. For reproducible results, it is crucial to maintain the cells in an optimal state throughout the entire workflow in order to achieve a consistent quality of the inoculum. To this end, it is helpful to develop a standardized protocol for the handling of the cells throughout the initial stages of process development. Several relevant factors are summarized in Figure 1. Establishing consistent culture conditions with narrow parameters can considerably improve reproducibility between individual runs.
Read more
Read less
Reproducible results require reproducible conditions inside the bioreactor; these depend on reliable sensor data which, in turn, depend on the precise calibration of the sensors. As an example, let’s take a look at the concentration of dissolved oxygen (DO). Polarization and calibration time of the DO-sensor may differ considerably depending on the user, which can potentially impact measurement results. Specialized software functions can help; for example, the function Auto Calibrate of the BioFlo® 320 and BioFlo 720 bioreactor control systems polarizes and calibrates the DO-sensors automatically, thus delivering reliable and reproducible results every time.
Dissolved oxygen, temperature, and pH are routinely measured in real time throughout the bioprocess. The integration of additional sensor types into bioprocess control enables capture of information regarding the condition of the cells or the concentration of nutrients and byproducts, and it also allows the implementation of feedback loops. This approach allows automation of, for example, culture feeding, based on the glucose concentration in the medium, and nutrient availability may be standardized.
In summary: reproducible conditions inside the bioreactor contribute to reproducible behavior of the cells, thus providing a solid basis for optimal process performance.
Tip 3: The right bioprocessing equipment
For cell culture processes, reproducibility is defined as equal behavior of replicates as well as comparable process behavior independent of scale. In both cases, bioprocessing equipment can help. On a small scale, during process development, parallel systems allow the simultaneous use of multiple bioreactors under identical conditions. Medium and inoculum originating from the same batches can be used to increase reproducibility between runs. The DASbox® Mini Bioreactor System , to name an example, allows parallel control of up to 24 vessels. The use of a family of instruments with consistent parameters is helpful during the scale-up process from small scale to pilot scale. Relevant factors include vessel geometry, material, and features.
First steps towards troubleshooting
If you notice that cell growth and the product yield of your bioprocess vary from day to day, it’s time to do some troubleshooting. It makes sense to first concentrate on the most obvious and the most accessible factors. One possible technical problem could be that sensors or gas lines were not connected properly. A reasonable subsequent step could include the analysis of process data, such as, for example, the supply of gases and liquids for the control of DO as well as pH; the feeding of media, as well as the various sensor data. A bioprocess software which offers deep and detailed insights into the performance of the bioreactor can help. Ideally, you will compare process data with historical runs, even if a novel process design is being evaluated.
Changes in the workflow also have the potential to alter results: for example, did I refrigerate my medium this time whereas previously I stored it at room temperature?
One final thought: is the system maintained on a regular basis? With increasing age, the quality of sensors and actuators will decline, which will lead to deviations. This effect can be minimized through regular calibration and adjustment.
Conclusion: there are many possible sources of error; it is important to have the right tools on hand to obtain sufficient high-quality data in order to be able to determine their cause.
To learn more read our ebook "Increasing the Reproducibility of Cell Culture Bioprocesses”:
Read more
Read less
Related links
Read more
Read less
Related documents
Read more
Read less