MENU
CA | CAD
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
CA | CAD
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
No results found
Search Suggestions

Webinar
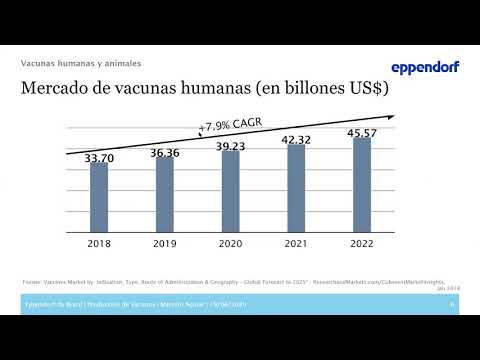
Production of vaccines – bioprocess development and scale-up
In these exceptional and unprecedented times, many companies are working eagerly on the development of a vaccine against COVID-19. New bioprocess technologies, such as single-use equipment and process automation, open up new possibilities for quality control and validation.
This is particularly important in GMP-regulated environments, such as in the development and manufacture of influenza vaccines. When facing a pandemic outbreak, developing new processes and rapidly increasing the volume of clinical production are essential for efficient development of new vaccines.
Date and time: These webinars took place on June 9 and 19, 2020.
Speaker: Marcelo Aguiar Msc., Zone Sales Director, Eppendorf do Brasil Ltda.
Languages: The recorded webinars are only available in Spanish.
Watch here the recordings of the webinars:
Date and time: These webinars took place on June 9 and 19, 2020.
Speaker: Marcelo Aguiar Msc., Zone Sales Director, Eppendorf do Brasil Ltda.
Languages: The recorded webinars are only available in Spanish.
Watch here the recordings of the webinars:
Read more
Read less
Videos not loading, because cookies have been rejected. Change your

Videos not loading, because cookies have been rejected. Change your