MENU
NO | NOK
NO | NOK
No results found
Search Suggestions
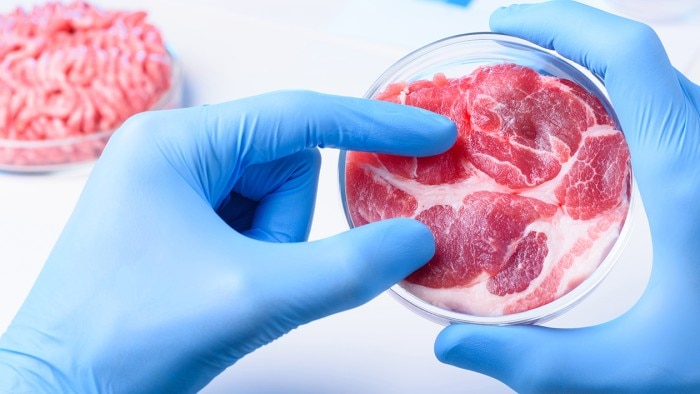
Cellular Agriculture – The Versatility of Stem Cells
- Cell Culture
- Stem Cells
Mark Post pioneered the creation of meat from stem cells and presented the world’s first hamburger from cultured beef. In this interview, the co-founder of Mosa Meat talks about his vision of growing meat in the lab.
Dr. Mark Post, MD, PhD, received his medical degree from the University of Utrecht in 1982 and, at the same University, his PhD in 1989. In his scientific career, he conducted research in the field of vascular biology and more specifically on neovascularization at the Royal Dutch Academy of Science and the Harvard Medical School, Boston, MA . Since 2002, Dr. Post was appointed as a professor for Vascular Physiology at the Maastricht University and of Physiology at the Maastricht University. His main research interests are vascular biology and tissue engineering of blood vessels and skeletal muscle. These subjects are studied from their basic molecular aspects and cellular mechanisms up to preclinical models and eventually, patients and consumers. In addition, he pioneered the creation of meat from stem cells and presented the world’s first hamburger from cultured beef in 2013. As a result, he was awarded the World Technology Award for solutions that benefit the environment at the World Technology Network summit in 2013. He recently co-founded and is CSO of Qorium and Mosa Meat, two start-ups respectively commercializing the technologies to produce bovine leather and cultured meat using tissue engineering.
Read more
Read less
Q: Where do you see the potential of stem cells outside of the clinical sector?
Mark Post: If you ask me, the biggest application for stem cells is obviously not going to be in the medical area. I'm a medical doctor by training, so I started thinking mostly about the medical applications. But I think the non-medical applications probably are going to be much bigger. I think the cellular agriculture applications are really exciting and have the potential to outgrow the medical applications by far. What we are doing now will not only have a social impact, e.g. how we see food or agriculture. It will also have a tremendous impact on the clinical sector and even kind of all the boundary conditions that the production system has to take into account.
Q: In which way will your research influence stem cell-based research and development?
Mark Post: The major challenge in working with stem cells are the high costs. The average working volume for cell and gene therapy applications is in the range of a few liters but can cost already around 200.000€. To produce enough cultivated meat in stirred-tank bioreactors we are talking of working volumes of several thousands of liters. If you start thinking about that problem and you start thinking about the scale that is required for the production, you immediately see that there is quite a disconnect from the medical field in terms of scale and in terms of cost considerations that you need to have. I believe that this will challenge all of us, especially companies such as the growth factor producers or things that are traditionally developed in the pharmaceutical industry or in Advanced Therapy Medicinal Products (ATMP) production.
All those concepts will be challenged by these cellular agricultural applications in terms of scale, cost, but maybe also really on how things are done. When you start to force yourself to work with a platform that is very costly already at small scale, but you are aiming for lower costs and way larger volumes, you need to start thinking about: How am I going to source the feedstock for the cells? How am I going to source the bioreactors for the cells? Do we really need to adhere to those stringent pharmaceutical criteria for all these components, or can we get this to work in a more food or feed grade system?
Q: Especially the costs are a major challenge on the way to commercialize lab grown food. One of your major focus is to reduce the costs of the growth media. How will this affect the medical field?
Mark Post: If you think of the costs of current gene therapy treatments with regulatory T-cells for example, these can lay in the range of 220,000€ per treatment, which is just crazy. I cannot really analyze the costs, but if you just think of the costs of the growth factors in the medium which are sold for a million euro per gram, whereas enzymes in the feed industry in a fermentation setting are produced for less than 10€ per gram. It is of course not the same protein, but it is a similar one, produced with the same technology. How can it be that one costs 1 million euro and one just 10? With our research, we are challenging that part of the industry. They have high-quality products and I believe that they have been doing the right thing, but they now see that they are going to have competitors who produce a very similar product with similar effectivity for one thousand of the price. Most of us will benefit from this development. Think of the costs of a regulatory T-cell therapy treatment – In a few years from now, this will cost only 10.000€ instead of 220.000€.
Q: Outside of the clinical sector, how can stem cells revolutionize, how we see food?
Mark Post: There is already a lot of technology in the food and agricultural industry, but people don’t necessarily realize this. Let’s take the example of milk: The milk you buy in a supermarket is seen by most people as the product that comes out of a cow, but of course, anybody who is working in the food and feed industry knows that the thing that comes out of the cow is just an ingredient of milk, and perhaps not even the most important one. With stem cells, or cellular agriculture, for instance, we are using recombinant proteins or stem cells to create a tissue that you can eat instead of having to grow in a cow. This is a scary concept for people, or at least an unfamiliar concept. But at some time, they will get familiar with it. In my vision, in 20–30 years from now, I believe that we will all eat meat that was primarily made in a setting outside of a cow, based on stem cell technology. This will enable us to implement a lot of standardizations, and recycling steps that we just cannot do with living organisms, which are mostly very inefficient in converting feed into proteins.
Mark Post: If you ask me, the biggest application for stem cells is obviously not going to be in the medical area. I'm a medical doctor by training, so I started thinking mostly about the medical applications. But I think the non-medical applications probably are going to be much bigger. I think the cellular agriculture applications are really exciting and have the potential to outgrow the medical applications by far. What we are doing now will not only have a social impact, e.g. how we see food or agriculture. It will also have a tremendous impact on the clinical sector and even kind of all the boundary conditions that the production system has to take into account.
Q: In which way will your research influence stem cell-based research and development?
Mark Post: The major challenge in working with stem cells are the high costs. The average working volume for cell and gene therapy applications is in the range of a few liters but can cost already around 200.000€. To produce enough cultivated meat in stirred-tank bioreactors we are talking of working volumes of several thousands of liters. If you start thinking about that problem and you start thinking about the scale that is required for the production, you immediately see that there is quite a disconnect from the medical field in terms of scale and in terms of cost considerations that you need to have. I believe that this will challenge all of us, especially companies such as the growth factor producers or things that are traditionally developed in the pharmaceutical industry or in Advanced Therapy Medicinal Products (ATMP) production.
All those concepts will be challenged by these cellular agricultural applications in terms of scale, cost, but maybe also really on how things are done. When you start to force yourself to work with a platform that is very costly already at small scale, but you are aiming for lower costs and way larger volumes, you need to start thinking about: How am I going to source the feedstock for the cells? How am I going to source the bioreactors for the cells? Do we really need to adhere to those stringent pharmaceutical criteria for all these components, or can we get this to work in a more food or feed grade system?
Q: Especially the costs are a major challenge on the way to commercialize lab grown food. One of your major focus is to reduce the costs of the growth media. How will this affect the medical field?
Mark Post: If you think of the costs of current gene therapy treatments with regulatory T-cells for example, these can lay in the range of 220,000€ per treatment, which is just crazy. I cannot really analyze the costs, but if you just think of the costs of the growth factors in the medium which are sold for a million euro per gram, whereas enzymes in the feed industry in a fermentation setting are produced for less than 10€ per gram. It is of course not the same protein, but it is a similar one, produced with the same technology. How can it be that one costs 1 million euro and one just 10? With our research, we are challenging that part of the industry. They have high-quality products and I believe that they have been doing the right thing, but they now see that they are going to have competitors who produce a very similar product with similar effectivity for one thousand of the price. Most of us will benefit from this development. Think of the costs of a regulatory T-cell therapy treatment – In a few years from now, this will cost only 10.000€ instead of 220.000€.
Q: Outside of the clinical sector, how can stem cells revolutionize, how we see food?
Mark Post: There is already a lot of technology in the food and agricultural industry, but people don’t necessarily realize this. Let’s take the example of milk: The milk you buy in a supermarket is seen by most people as the product that comes out of a cow, but of course, anybody who is working in the food and feed industry knows that the thing that comes out of the cow is just an ingredient of milk, and perhaps not even the most important one. With stem cells, or cellular agriculture, for instance, we are using recombinant proteins or stem cells to create a tissue that you can eat instead of having to grow in a cow. This is a scary concept for people, or at least an unfamiliar concept. But at some time, they will get familiar with it. In my vision, in 20–30 years from now, I believe that we will all eat meat that was primarily made in a setting outside of a cow, based on stem cell technology. This will enable us to implement a lot of standardizations, and recycling steps that we just cannot do with living organisms, which are mostly very inefficient in converting feed into proteins.
Read more
Read less

Stirred-tank bioreactor system for scalable cell expansion
Q: What are the challenges and limiting factors in the development of lab grown meat?
Mark Post: It will take a lot of more time and efforts to bring lab grown meat to the market. One of the major challenges will be the scaling issue. If you think of cell therapy applications, working volumes in the low liter range are sufficient, but one hamburger has 10 billion cells. We need to scale up the production tremendously and this process needs to be very robust and industrial. This is the point where bioreactor design and process optimization come into play. There’s a need for technologies to develop single cell suspension or maybe modified planar systems like packed-bed bioreactors. So far, working in these large volumes where you harvest the cells has never been done in that scale with mammalian cells.
The yield of course depends on the achieved cell density per mL in the bioreactor, which is currently in a range of 10.000.000 cells per mL, but could be even higher if the cells are grown as aggregates. Despite of the scaling issue, there are innovations missing for the large scale-differentiation of the stem cells. The end-product consists of differentiated cells which already are in the form of tissues. You want to define how your tissue is shaped, how big it is, and how it is being made. For this, you need customized solutions, which are not available on the market yet. And this part also needs to be automated, so you need to think about automated tissue dispensing, whether this is 3D printing or extrusion or basically injecting and assembling that at high density into a bioreactor.
Q: Do products of the cellular agriculture fall under special regulations?
Mark Post: In Europa it is considered a novel food, which means that safety has to be demonstrated and accepted. This is uncharted territory. Nobody has ever done that, also the European Food Safety Authority (EFSA) has never done this before. They also do not exactly know exactly what they need to do and what they need to know. But fortunately, Europe has defined the pathway of regulatory approval quite extensively and with that are ahead of most other nations. The regulatory guidelines mostly focus on product composition and on the process itself. The production process needs to be described and defined. But there will be additional questions since everyone in this field is learning right now.
Mark Post was interviewed by Eppendorf in 2020.
Mark Post: It will take a lot of more time and efforts to bring lab grown meat to the market. One of the major challenges will be the scaling issue. If you think of cell therapy applications, working volumes in the low liter range are sufficient, but one hamburger has 10 billion cells. We need to scale up the production tremendously and this process needs to be very robust and industrial. This is the point where bioreactor design and process optimization come into play. There’s a need for technologies to develop single cell suspension or maybe modified planar systems like packed-bed bioreactors. So far, working in these large volumes where you harvest the cells has never been done in that scale with mammalian cells.
The yield of course depends on the achieved cell density per mL in the bioreactor, which is currently in a range of 10.000.000 cells per mL, but could be even higher if the cells are grown as aggregates. Despite of the scaling issue, there are innovations missing for the large scale-differentiation of the stem cells. The end-product consists of differentiated cells which already are in the form of tissues. You want to define how your tissue is shaped, how big it is, and how it is being made. For this, you need customized solutions, which are not available on the market yet. And this part also needs to be automated, so you need to think about automated tissue dispensing, whether this is 3D printing or extrusion or basically injecting and assembling that at high density into a bioreactor.
Q: Do products of the cellular agriculture fall under special regulations?
Mark Post: In Europa it is considered a novel food, which means that safety has to be demonstrated and accepted. This is uncharted territory. Nobody has ever done that, also the European Food Safety Authority (EFSA) has never done this before. They also do not exactly know exactly what they need to do and what they need to know. But fortunately, Europe has defined the pathway of regulatory approval quite extensively and with that are ahead of most other nations. The regulatory guidelines mostly focus on product composition and on the process itself. The production process needs to be described and defined. But there will be additional questions since everyone in this field is learning right now.
Mark Post was interviewed by Eppendorf in 2020.
Read more
Read less
