MENU
CN | CNY
CN | CNY
-
- All Centrifuges
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Sample and Information Management
- GLP Products China
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Pipette Tips
- Bottle-Top Dispensers
- Pipette Controllers
- Dispenser & Pipette Accessories
- Automated Pipetting
- Automation Consumables
- Automation Accessories
- Liquid Handler & Pipette Services
Sorry, we couldn't find anything on our website containing your search term.
Sorry, we couldn't find anything on our website containing your search term.
- Home
- Products
- Bioprocess
- Bioprocess Equipment for Commercial Manufacturing
- BioBLU® f HNQ Single-Use Bioreactors
BioBLU® f HNQ Single-Use Bioreactors
Product Information
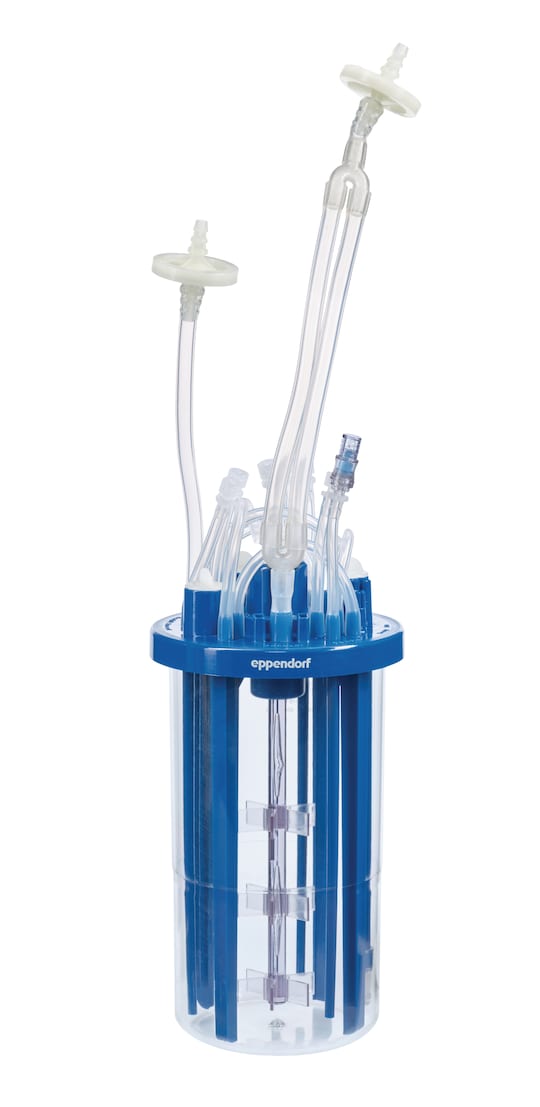
BioBLU f HNQ Single-Use Bioreactors are rigid- walled stirred-tank bioreactors suitable for use in clincal and commercial manufacturing. They are specifically designed for robust microbial applications using bacteria, yeasts, and fungi. The scalable BioBLU f HNQ portfolio covers working volumes up to 3.75 L. The rigid wall stirred tank design mimics traditional bioreactors and supports effective mixing and mass transfer making them suitable for high-cell density fermentation.-
Single-Use Bioreactors can help you increase efficiency and minimize process risks. Find out more!
-
Discover the BioBLU f Single-Use Bioreactors for R&D.
You will find additional download material at the bottom of this page
Single Devices (2)
2 Single Devices
Catalog no.
1386115000
|
Catalog no.
1386128000
|
Show more Products
Added to Your Cart
Product Information
Applications
Features
Product Information
HNQ stands for Harmonized for Next-Generation Quality, highlighting our commitment to delivering products that carry harmonized certificates and Validation Guide documents. The BioBLU HNQ series represents a leap forward in customer facing quality documentation, supporting our customers' equipment qualification and process validation activities.
The BioBLU f HNQ Single-Use Bioreactors for microbial applications at a glance:
The rigid wall stirred tank design mimics traditional bioreactors and supports effective mixing and mass transfer making them suitable for high-cell density fermentation.
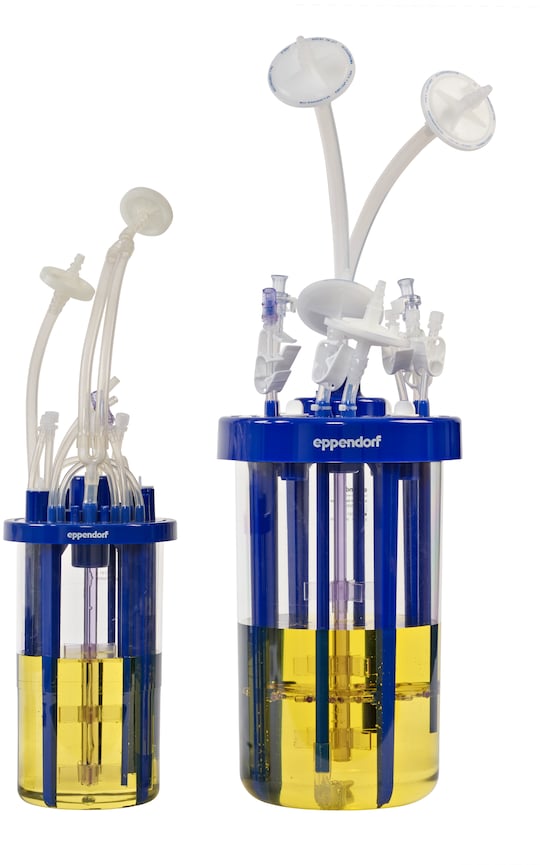
• BioBLU 1f HNQ (working volume 320 mL – 1.25 L)
• BioBLU 3f HNQ (working volume 1.25 L – 3.75 L)
Suitable for use in clinical and commercial manufacturing
• BioBLU HNQ Single-Use Bioreactors are available with LOT-specific Validation Guide, irradiation certificate, and the sterile- and endotoxin test report.
• We offer a unified solution package of bioprocess controllers, bioreactors, bioprocess SCADA software, and services that together support compliance of bioprocesses with GMP requirements.
Warning! Not approved for applications in medical diagnostics or therapy! The single-use vessel has not been developed for applications in medical diagnostics or therapy. It is not medical equipment within the meaning of Regulation (EU) 2017/745. Do not use the single-use vessel for medical or therapeutic applications.
Eppendorf Bioprocess Products – application in GMP regulated environment
Download document to find out which features you can expect at minimum from Eppendorf products that are deemed suitable or compatible for GMP.
The BioBLU f HNQ Single-Use Bioreactors for microbial applications at a glance:
The rigid wall stirred tank design mimics traditional bioreactors and supports effective mixing and mass transfer making them suitable for high-cell density fermentation.
• BioBLU 1f HNQ (working volume 320 mL – 1.25 L)
• BioBLU 3f HNQ (working volume 1.25 L – 3.75 L)
Suitable for use in clinical and commercial manufacturing
• BioBLU HNQ Single-Use Bioreactors are available with LOT-specific Validation Guide, irradiation certificate, and the sterile- and endotoxin test report.
• We offer a unified solution package of bioprocess controllers, bioreactors, bioprocess SCADA software, and services that together support compliance of bioprocesses with GMP requirements.
Warning! Not approved for applications in medical diagnostics or therapy! The single-use vessel has not been developed for applications in medical diagnostics or therapy. It is not medical equipment within the meaning of Regulation (EU) 2017/745. Do not use the single-use vessel for medical or therapeutic applications.
Eppendorf Bioprocess Products – application in GMP regulated environment
Download document to find out which features you can expect at minimum from Eppendorf products that are deemed suitable or compatible for GMP.


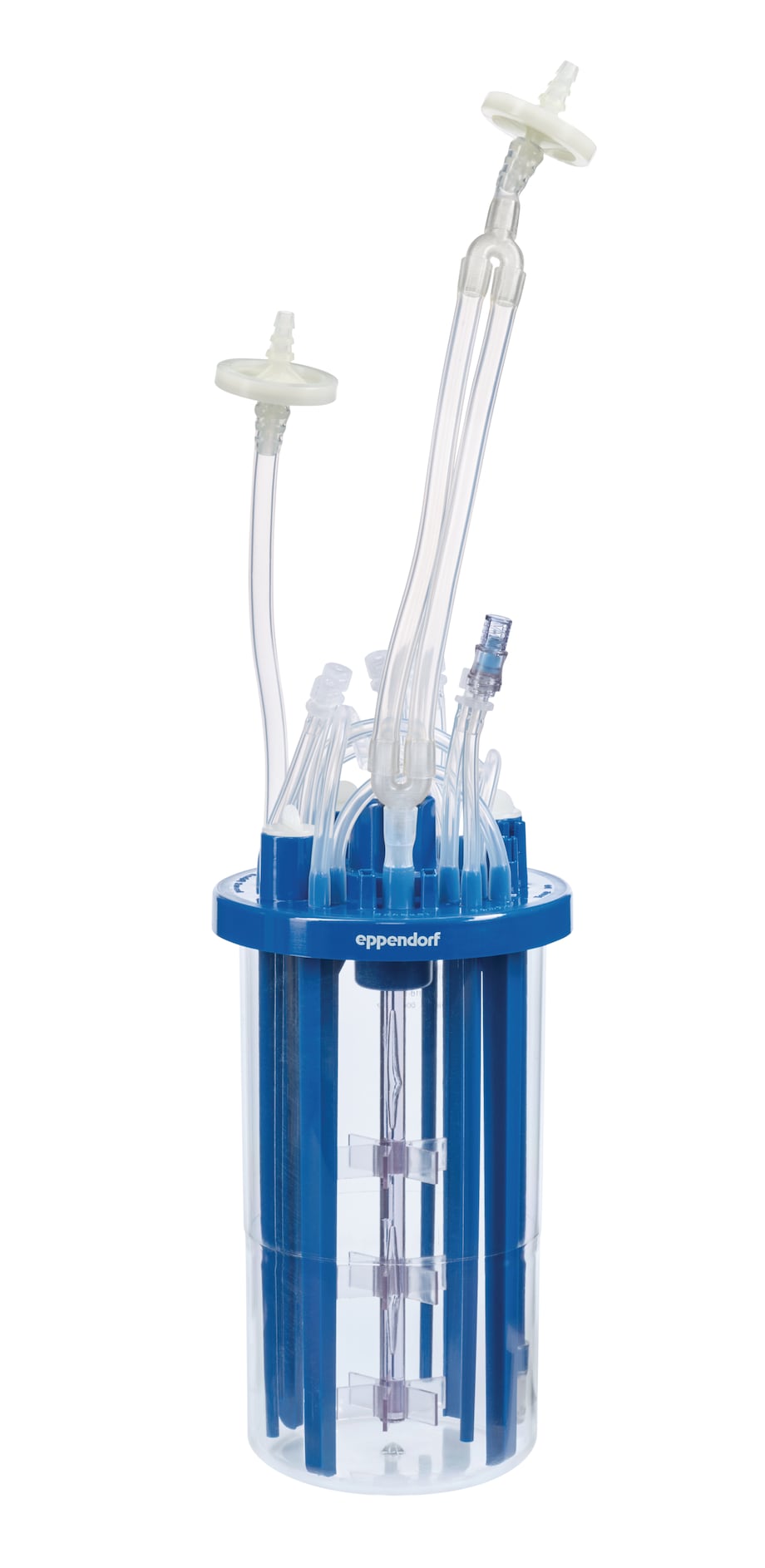
Bioreactor body and head plate
• Stirred-tank design: The stirred-tank design offers reliability scalability and facilitates the easy replacement of traditional glass bioreactors.
• Equipment: BioBLU Single-Use Bioreactors are assembled with sparger, overlay, gas filters for inlet and exhaust as well as penetrations for pH, DO, and temperature sensors, liquid additions, sampling, and harvest. All head plate ports are clearly marked for ease of use. Spare Pg 13.5 ports can be equipped with single-use septa, tri-ports and compression fitting adaptors for more flexibility.
• Sterilization: BioBLU 1f HNQ Single-Use Bioreactors are delivered sterile. Irradiated by > 25 kGy (X-ray). SAL-level 10-6 for X-ray irradiated BioBLU Single-Use Bioreactors. The BioBLU 3f HNQ Single-Use Bioreactor is not presterilized and is autoclavable.


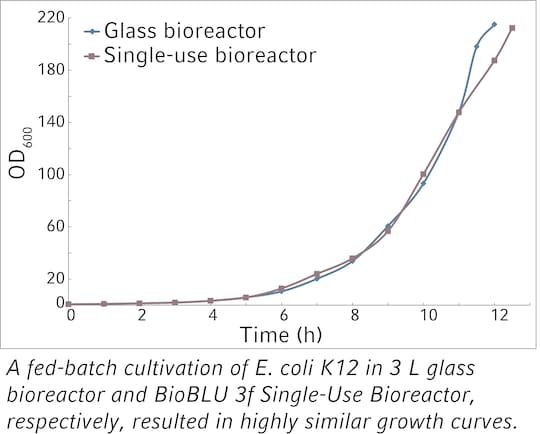
Suitable for high cell densities
BioBLU f HNQ Single-Use Bioreactors support the high mass transfer and heat removal requirements of fermentation processes.
• Motor: Powerful overhead drives enable high agitation speeds. Sealed magnetic drive with fully enclosed bearings maintain vessel sterility.
• Baffles: Interior baffles aid mixing and mass transfer. The baffles of the BioBLU 1f HNQ additionally provide efficient heat removal through active cooling.
• Impellers: Multiple Rushton-type impellers for efficient mixing and mass transfer.
• Cooling: Smart solutions for cooling using cooling baffles or cooling fingers.
• Exhaust treatment: Effective liquid-free exhaust condensation (Peltier), electric heater band or water-cooled exhaust treatment.


Read More
Read Less
Applications
- Cultivation of bacteria, yeasts, and fungi.
- Suitable for high-cell density fermentation.
- For the cultivation of human and animal cells we offer the BioBLU c HNQ Single-Use Bioreactor product line.
Features
- Single-use, stirred-tank, rigid-walled bioreactors available in sizes ranging of up to 3.75 L working volume
- Designed for high-density fermentation processes
- Multiple Rushton-type impellers for efficient mixing and mass transfer
- Sealed magnetic drive with fully enclosed bearings maintain vessel sterility
- Baffles for excellent mixing and mass transfer
- Bioreactors are assembled with sparger, gas filters for inlet and exhaust as well as penetrations for pH, DO, temperature, liquid additions, sampling, and harvest
- For use with Eppendorf bioreactor systems
Downloads: BioBLU® f HNQ Single-Use Bioreactors
You might also consider