MENU
TH | THB
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
TH | THB
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.
Sorry, we couldn't find anything on our website containing your search term.
Leachables and Extractables in Bioprocessing
Lab Academy
- Health & Medicine
- Cell Culture
- Quality
- Services
- Bioprocess
- Essay
Day-to-day bioprocess development is a special challenge. Here, we give practical tips at hand, starting with a discussion about the influence of leachables and extractables on product safety and how to mitigate these issues. Also, we give an overview on impeller types and how they affect mixing in a stirred-tank bioreactor.
Finding the needle in the haystack: Leachables and extractables in bioprocessing
The influence of leachables and extractables (L&E) on drug quality and safety is one of the hot topics in current biopharmaceutical processing. The L&E question, especially regarding packaging, has been discussed for years. However, with the increased use of single-use bioreactor equipment, the L&E debate touches on upstream bioprocessing as well.L&E – What are we talking about?
Plastics are used throughout the supply chain, production, and packaging of drugs. Examples include single-use bioreactors, storage containers, disposable purification equipment, and primary packaging systems. The concern is that chemicals, organic or inorganic, could leach from the plastics into solution in liquid pharmaceutical products. We therefore call these potential contaminants leachables. In biopharmaceutical production, leaching problems are most likely to occur in the bioprocessing of suspension cultures, the downstream processing of proteins, and the final packaging of liquid formulations.Read more
Read less
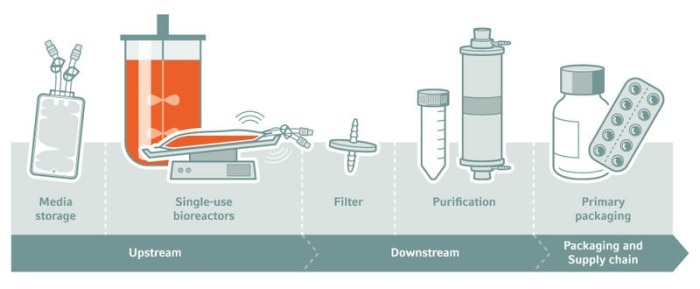
Sources of L&E in biopharmaceutical production processes
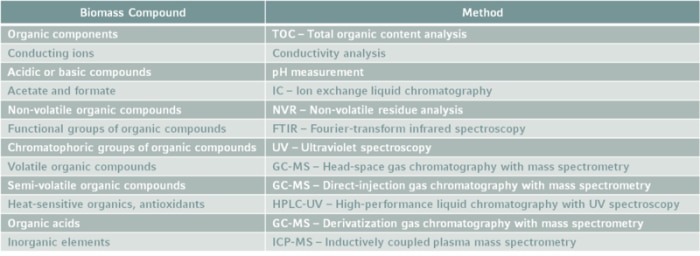
Although the terms “leachables” and “extractables,” are often used in the same breath, as with the contraction, “L&E,” we need to think of them separately. Leachable compounds would typically only be present in trace quantities, so determining which compounds are present and then quantifying them analytically are very challenging—thus the expression “finding the needle in the haystack” in the title of this article. Experience has shown that it is often best to first identify the chemical species that might possibly be leached by subjecting each plastic used in the system to a rigorous process of extraction in the laboratory. Unlike leachability testing, which usually occurs under the actual production condition, storage system, or packaging, extraction tests are conducted in the laboratory without constraints—temperatures and pressures may be raised, strong acids or organic solvents added, and multiple cycles conducted—to force any possible contaminants to identify themselves. The extractables thus elicited tell us what “needles to look for in the haystack” using highly specific and sensitive analytical techniques. Substances commonly identified by extractability testing include volatiles, semi-volatiles and non-volatiles, acidic or basic compounds, inorganic elements, and conducting ions.
Read more
Read less
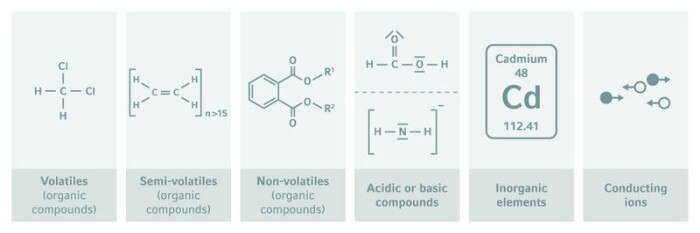
Common L&Es
How to deal with L&Es
Chemicals that leach into pharmaceuticals may affect the health of patients, and thus the safety of the drug. Two types of factors influence the amount and impact of L&Es in final pharmaceutical formulations: process-related and dose-related. Process factors, for example, include the contact time and contact area per volume of the pharmaceutical product with the plastic material. Process factors determine the concentration of leachables in the drug product. For drug safety, however, the leachable concentration is less important than the dosage the patient receives, and its frequency.In a jungle of no regulations
There are no global regulatory guidelines that specify how to deal with L&Es in biopharmaceutical processing. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) only stipulate that process material should not “present any hazards to the product” and should not be “reactive, additive, or adsorptive”. There are neither binding guidelines for testing and evaluation nor any permissible limits.Several organizations, however, are currently working together to establish best practices and guidance. The Bio-Process Systems Alliance (BPSA) works out L&E testing strategies in the English-speaking countries, while the Bioprocess Technology Working Group at DECHEMA is doing so in the German-speaking countries. [1] Both BPSA and DECHEMA recommend the implementation of L&E testing programs at an early stage of bioprocess development. The USP is expected to soon publish its new chapter <665> “Polymeric Components and Systems Used in the Manufacturing of Pharmaceutical and Biopharmaceutical Drug Products” (draft published as <661.3> [2]).
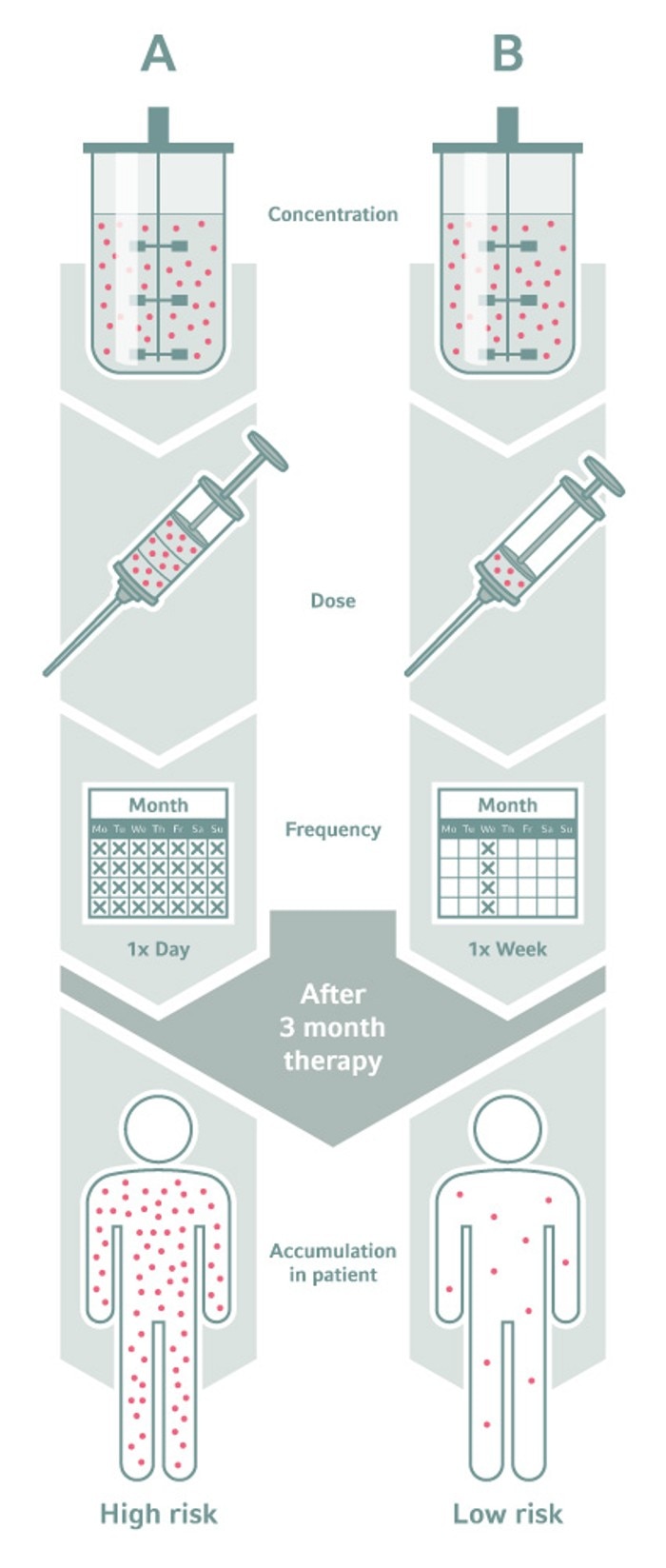
Dosage Dependance – The safety risk of L&Es in final drug formulations strongly depends on dosage and taking frequency.
Getting started with proper testing strategies
Before single-use equipment became common in biopharmaceutical production, L&Es were mainly considered for packaging. Today, most biomanufacturers use simple biological reactivity tests to evaluate the risk of L&E impurities. This is insufficient. Modern systems require comprehensive and meaningful analytics. [3]“Searching for leachables without a prior extractable study is like looking for a needle in a haystack”, is how Desmond Hunt, the principal scientific liaison at the US Pharmacopeial Convention, was recently cited in an issues paper. [4] This quote sums up the challenges and requirements of L&E testing.
A proper testing strategy should be based on extractable data, as this provides the needed primary information on potentially leachable chemical entities. Compared to determining leachables it is quite fast and easy to search for extractables for plastic components used in a biopharmaceutical production process.
Read more
Read less
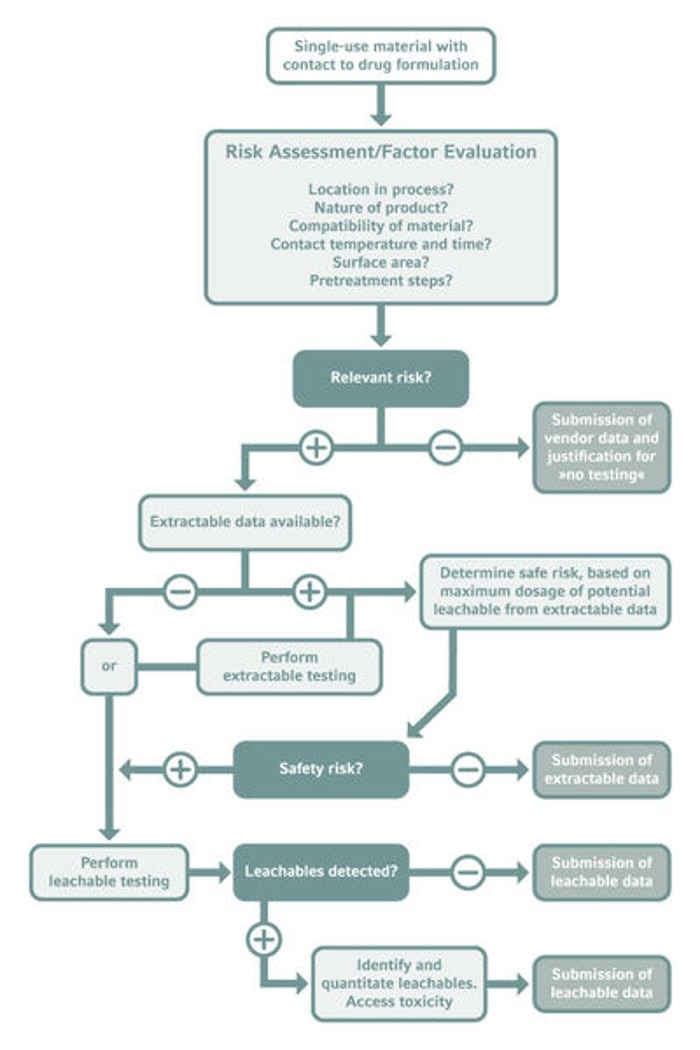
The first step regarding L&Es in drug quality assurance is development of a comprehensive risk assessment strategy if there is any contact between the drug formulation and plastic components. [5] The specific risk factor for leaching should be evaluated for each material contacted. Vendor data can be a first reference here. Not only the raw material origin, but also how it is processed and pre-treated play a major role to evaluate the risk for L&Es. For example, softeners or other additives may cause leaching of components. Also, gamma ray-based sterilization of plastic materials is discussed to make polymer layers degrade which is why other methods might be preferable.
Whenever a potential risk is identified, the extractable data for this contact material must be analyzed and the drug formulation needs to be tested for all possible leachable compounds.
Whenever a potential risk is identified, the extractable data for this contact material must be analyzed and the drug formulation needs to be tested for all possible leachable compounds.
Read more
Read less
An issue for teaming
Most experts agree that an effective testing strategy for L&Es must be based on a deep understanding of the entire process. This would need to include R&D studies, process descriptions, batch records, standard operating procedures, technical reports, batch testing, data trending, and operation parameters.Read more
Read less