-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.

Cell seeding protocol – Guide on how to seed cells correctly
Lab Academy
- Cell Biology
- Cell Culture
- Lab Routine
- Pipetting & Dispensing
- Reproducibility
- Pipettes
- Pipette Tips
- Essay
Cell seeding is usually the first protocol step and a standard procedure in cell-based experiments. A correct and standardized cell seeding protocol is a critical factor for reproducible experimental results. The main challenge in this step is to achieve and maintain comparable cell numbers in all repeated experiments.
Dr. Jessica Wagener, Application Specialist Cell Handling at Eppendorf
Contents
- The impact of standardized cell seeding protocol
- The time factor and cell sedimentation
- Video: Correct pipetting technique
- Air bubble formation
- Video: How to avoid air bubble formation in cell seeding
- Homogeneous cell adhesion
- Cell density dependent behavior
Why is cell seeding a critical step in all cell culture experiments?
Cell seeding is usually the first protocol step and a standard procedure in cell-based experiments. A correct and standardized cell seeding protocol is a critical factor for reproducible experimental results. The main challenge in this step is to achieve and maintain comparable cell numbers in all repeated experiments. Variations in seeding cell numbers and the formation of air bubbles during seeding will increase standard deviations, making your results less reliable. Therefore, several factors need to be considered when establishing a solid cell seeding protocol in your lab.
The time factor in cell seeding protocols
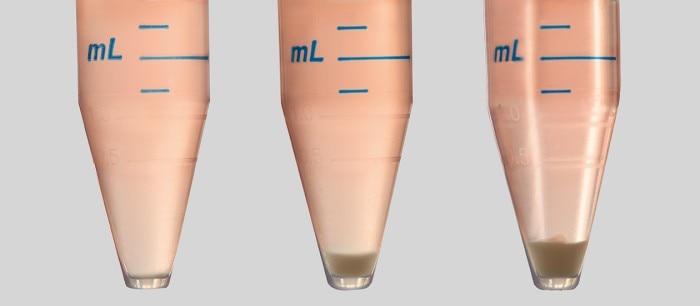
When you seed your cells, from a 15 mL tube into a multi-well plate, for example, it will take some time until you have filled all the wells. No matter if you use a single-channel or a multi-channel pipette in your cell seeding protocol, the longer the process takes, the more cells will sediment in the tube. So, without mixing the cell suspension in the tube or reservoir, you will get varying cell numbers from one well to another (Image 1). Read more
Read less
Video: Correct pipetting technique in a cell seeding protocol
Read more
Read less
Videos not loading, because cookies have been rejected. Change your

Air bubble formation during cell seeding
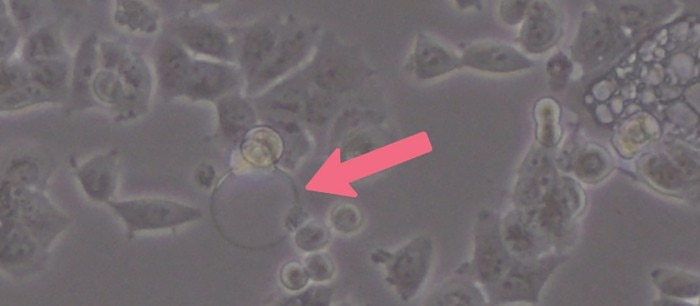
Cell culture media tend to foam due to the high protein content of supplemented serum. Air bubbles can hinder cell attachment during seeding and therefore create well-to-well variations of cell numbers (Image 2). Especially in smaller plate formats like 96- and 384-well plates, air bubbles can have a big impact due to the small growth area per well. So, although resuspending or mixing is necessary, you should not overdo it and avoid too much pipetting to reduce air bubble formation. Gentle pipetting also reduces shear forces and stress for your cells – a critical part of every cell seeding protocol.Read more
Read less
Video: How to avoid air bubble formation in cell seeding
Read more
Read less
Videos not loading, because cookies have been rejected. Change your

Homogeneous cell adhesion
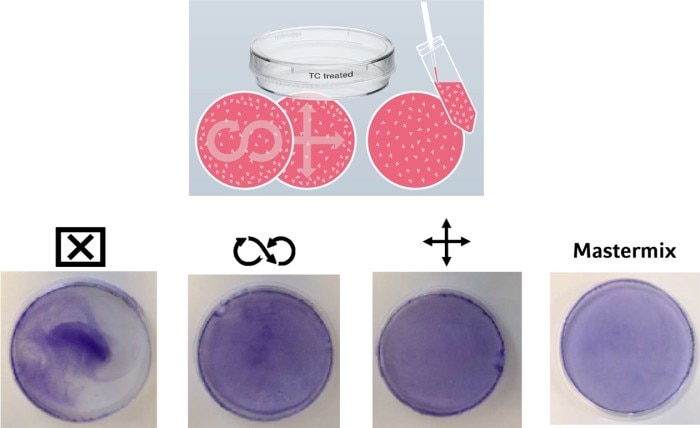
In addition to an equal cell number, a homogeneous cell distribution on the growth surface of a culture vessel can have an impact on your experimental outcome. Different techniques are common, usually passed from more experienced scientists in a lab to their trainees. But what is the best method to evenly distribute the cells on the growth surface? In Image 3, you can see a comparison of different techniques.On the left, you see what happens if you just prefill the vessel with cell culture medium, add the cells, and call it a day. As a result, the cells mainly adhere in the middle of the vessel. To achieve an equal cell distribution, some people use a “figure-eight” movement, while others prefer a cross-like movement of the plate or dish. Both techniques lead to a better distribution of the cells. However, the smaller the vessel diameter, the less movement of the liquid you have, leading to an uneven distribution of cells. An effective way to circumvent this effect during cell seeding is to first dilute the cell suspension to the desired concentration in a tube or reservoir (mastermix) and then pipette the final volume to the culture vessel in one step.
Read more
Read less
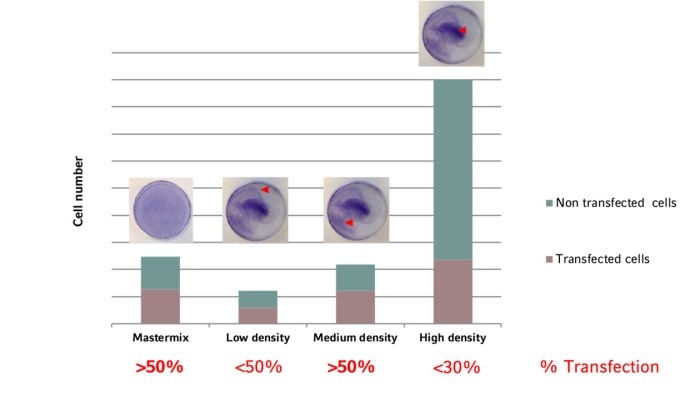
Does cell density matter?
The uniformity of cell distribution can have a significant impact on your experiment. Of course, there are cell types that tend to grow in colonies rather than forming a homogenous monolayer. But in general, the cells should spread as uniformly as possible over the entire growth surface of the culture vessel. One example showing the direct impact of cell distribution and cellular responses is the transfection efficiency (image 4 and 5).Read more
Read less
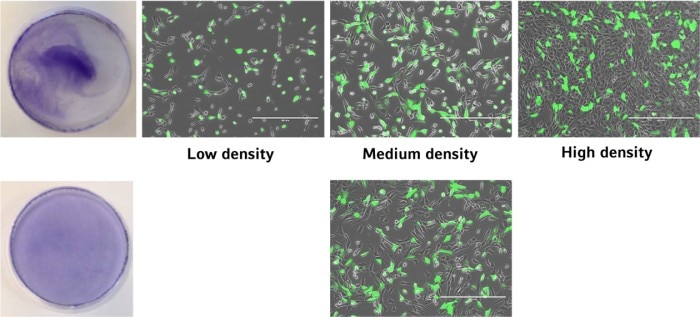
Read more
Read less