MENU
SK | EUR
SK | EUR
No results found
Search Suggestions
Eppendorf Biopur®
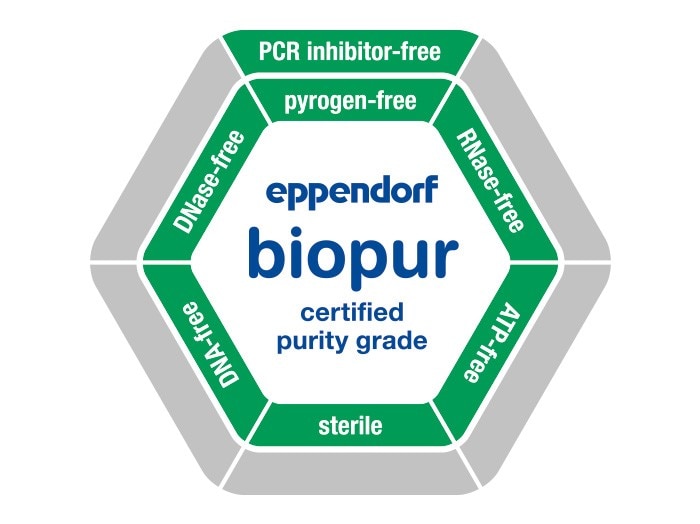
To meet the highest demands of the medical, pharmaceutical and food industries in a manner comparable to that of molecular biology and cell technology, the quality and purity standard Eppendorf Biopur was defined. All consumables available in this highest possible degree of purity are guaranteed to be sterile and free of* pyrogens, RNases, DNases, DNA and ATP as well as PCR inhibitors. This purity is ensured by an elaborate, automated manufacturing process which safeguards the product against any contamination with biological substances.
The advantages of this purity grade are obvious:
The advantages of this purity grade are obvious:
- Safety guaranteed, even for the most sophisticated applications
- Reduced costs - inconclusive, usually expensive tests no longer necessary
- "Ready for use" products reduce labor and time required
- Individually blistered consumables and “micro batches” in re-sealable boxes provide effective protection against soiling or contamination
Read more
Read less
Which requirements does Eppendorf Biopur® fulfill?
- Certified sterility: Each lot is sterilized by irradiation (in accordance with ISO 11137) or ethylene oxide gassing (in accordance with ISO 11135). Each lot of sterile products is tested for sterility. The testing procedures for sterility testing comply with the requirements of Ph.Eur. 2.6.1 (European Pharmacopeia) and USP (United States Pharmacopeia).
- Certified free of pyrogens: Each lot of Biopur products is tested for the absence of* endotoxins and thus demonstrated as being free of pyrogens. In order to meet the international requirements for the absence of pyrogens, Eppendorf uses the kinetic-turbidimetric LAL test (Ph. Eur. 2.6.14).
- Certified free of RNases, DNases, PCR inhibitors
- Testing each lot for the absence of DNase and RNase: Reaction buffers with one DNA or one RNA conductor are incubated at 37°C in the consumables for several hours. There must be no trace of DNA or RNA digestion.
- Each lot is tested for the absence of PCR inhibitors in a separate PCR experiment against a standard reaction.
- Certified free of human and bacterial (E. coli) DNA
- The absence of human and bacterial DNA is verified in each lot by PCR analysis. The detection limit is < 2 pg DNA. This quantity is smaller than a single human cell
- Certified ATP-free*
- The test procedure for the quantitative and qualitative detection of ATP is already an integral part of hygiene monitoring, e.g., in the pharmaceutical industry. Non ATP-free lab products would be indicated immediately in this very sensitive test.
- Furthermore, all Eppendorf Biopur products fulfill the quality standards defined for our standard consumables. The requirements regarding wetting behavior, liquid tightness, accuracy, chemical resistance, etc. are met in full.
Only those consumables that have passed all the necessary tests shall be labeled “Biopur”, a quality seal which assures customers of the suitability of their Eppendorf consumables for direct use in experiments and analysis without the need for further treatment. Each lot is tested and certified by an external accredited laboratory as a matter of course.
The lot-specific certificates are available on request and on our website.
Read more
Read less
*The suffix or word „-free/free of“ or "absence of" in the context of the purity grades means, testing showed conformity within the detection limits.
Read more
Read less
To our products
Products available in Biopur® are e.g.:
Read more
Read less