-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
Liquid Handling FAQ
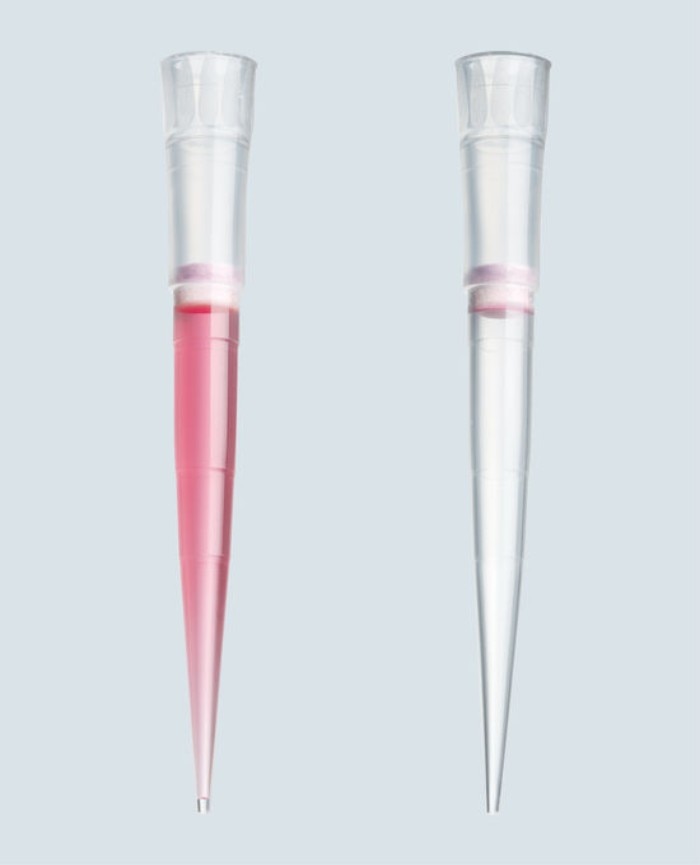
01. How can I avoid dripping of the pipette when using ethanol?
The pipet tip tends to drip when liquids like ethanol, acetone or chloroform are used. The cause of the dripping is the high vapor pressure of the liquid that leads to an expansion of the air cushion inside manual pipettes. One way to avoid dripping is pre-wetting the pipet tip. You have to aspirate and dispense the liquid 2-3 times prior to transferring the liquid into your destination vessel. Another option is to use a different pipetting system, the so called positive displacement system. It is without an air cushion and therefore no dripping occurs.
Related files

02. How can I pipette glycerol?
Eppendorf solutions:
Eppendorf ViscoTip®
Read more
Read less
Videos not loading, because cookies have been rejected. Change your

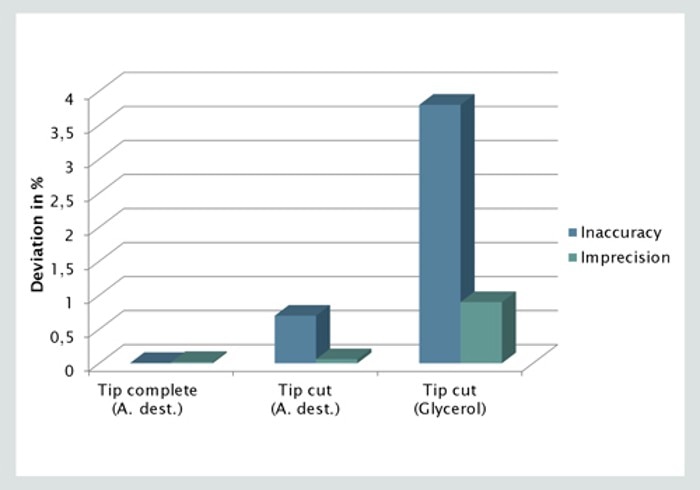
03. Is it okay to cut the pipet tip prior to transferring glycerol?
No, cutting the pipet tip is the worst thing to do when transferring glycerol. The pipetting result will neither be precise, nor accurate. The pipet tip is deformed and the orifice will be frayed so that plastic residues might also fall into the sample. It is better to use a full pipet tip and reverse pipetting technique or a positive displacement system.
Eppendorf solutions:
Eppendorf ViscoTip®
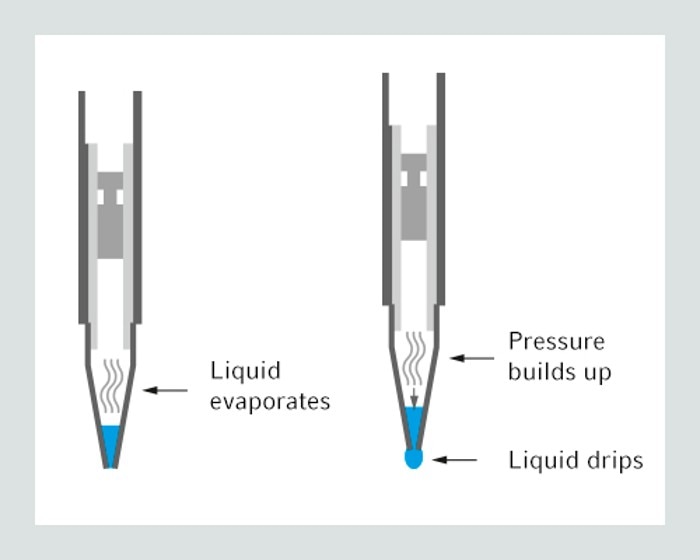
04. How can I avoid foam formation inside my pipette?
Some liquids contain a large amount of protein, like BSA-solution or cell culture medium. These tend to build up foam during pipetting, especially in the moment of blow-out when air is introduced into the sample. To avoid this phenomenon one should apply reverse pipetting technique with air cushion pipettes. Another option is the usage of positive displacement systems. These lack the air cushion and therefore no foam can build up during dispensing of such liquids.

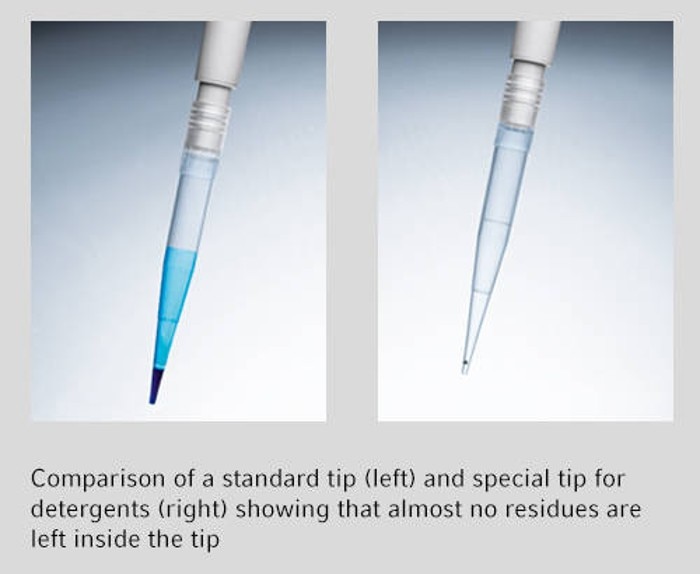
05. How can I pipet detergents?
Pipetting detergents such as Triton X-100 or Tween 20 lower the surface tension of aqueous solutions. Furthermore they tend to stick to the inside of a pipet tip after dispensing. That means that never all the liquid inside the pipet tip is dispensed, leading to sample loss, inaccurate volume delivery and in the long run higher reagent costs. Efficient pipetting of detergents is only possible with pipet tips specifically developed for these liquids or a positive displacement system.
Eppendorf solutions
06. Why do aerosols bear a risk of contamination?
Aerosols can be formed by almost every liquid. If the air humidity inside the pipette is below liquid humidity small microdrops evaporate upwards into the pipet tip and up into the pipette cone. If the liquids used are warm e.g., cell culture medium, the evaporation increases. The inside of the pipette cone can suffer from corrosion over time. But even worse is the risk of contamination brought into samples via aerosols. If an infectious liquid or bacterial culture evaporates into the pipette cone you will dispense the infected and contaminated aerosols with the blow-out. It is possible that you cross-contaminate multiple samples. Preventing aerosols is necessary and can be achieved by using high quality two-layered filter tips that block fine aerosols, bacteria and viruses. If you are using highly infectious liquids a positive displacement system might be more advantageous because the whole sample is enclosed in the tip without any contact to the liquid handling instrument.

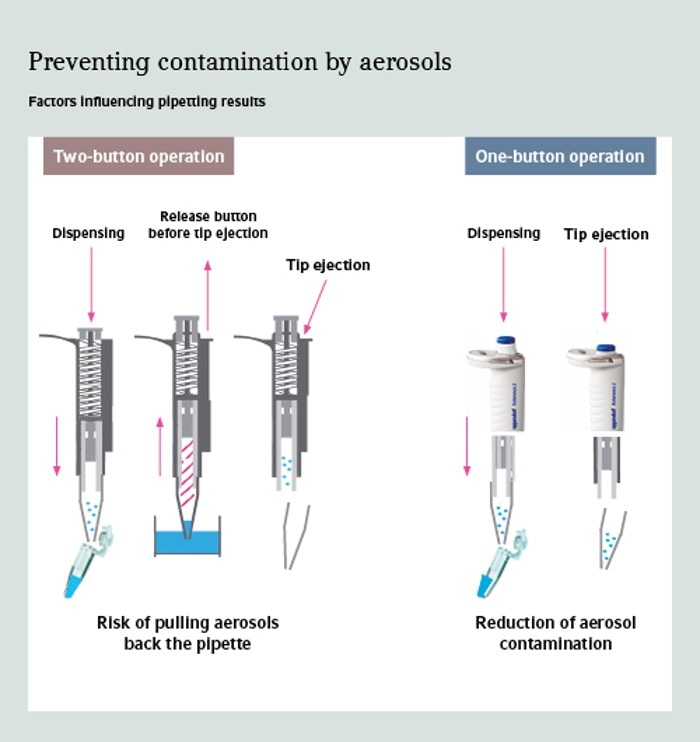
07. Why does a one-button operation pipette leads to less pipette contamination?
When using a two-button pipette you have to aspirate and dispense the liquid with one button. Then you switch over to a second button for tip ejection. Liquid aerosols ascend up into the pipet tip and even higher into the pipette cone during aspiration. Then the liquid is dispensed and you aspirate air again when releasing the operation button. Thereby you may aspirate the aerosols again, prior to tip ejection via the second button. With a one-button operation pipette you aspirate and dispense the liquid and then eject the tip in the same downwards movement. So you do not aspirate aerosols back into your pipette.
08. What is most important for an accurate pipetting result?
1. Hold the pipette vertical during aspiration
2. Immerse the pipet tip only as much as needed into the liquid (~ 2-3 mm)
3. Release the operation button slow and calm
4. Rest the pipet tip ~ 2 sec in the liquid before pulling the pipette up
5. Dispense at a 20-45° angle with contact to the vessel wall
6. Dispense at a calm and moderate speed
7. Perform the blow-out
Read more
Read less
Related files

09. Why do I have to calibrate a pipette?
Read more
Read less
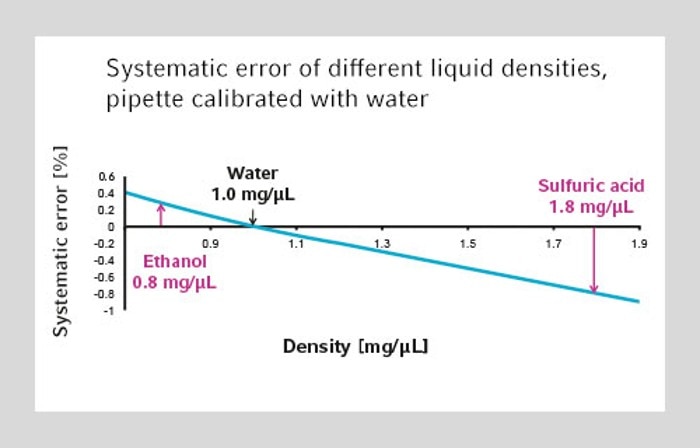
10. Why do I have to adjust a pipette for high density liquids?
Liquids with a high density such as sulfuric acid or phosphoric acid have a tendency to pull down the air-cushion inside the pipette. Thereby the air-cushion is elongated which leads to lower volume aspiration than set on the pipette. The pipetting result is always inaccurate without having the pipette adjusted to the higher density. After adjustment the pipette aspirates a little bit more liquid due to a smaller air-cushion. The pipetting result is correct then.
Related files

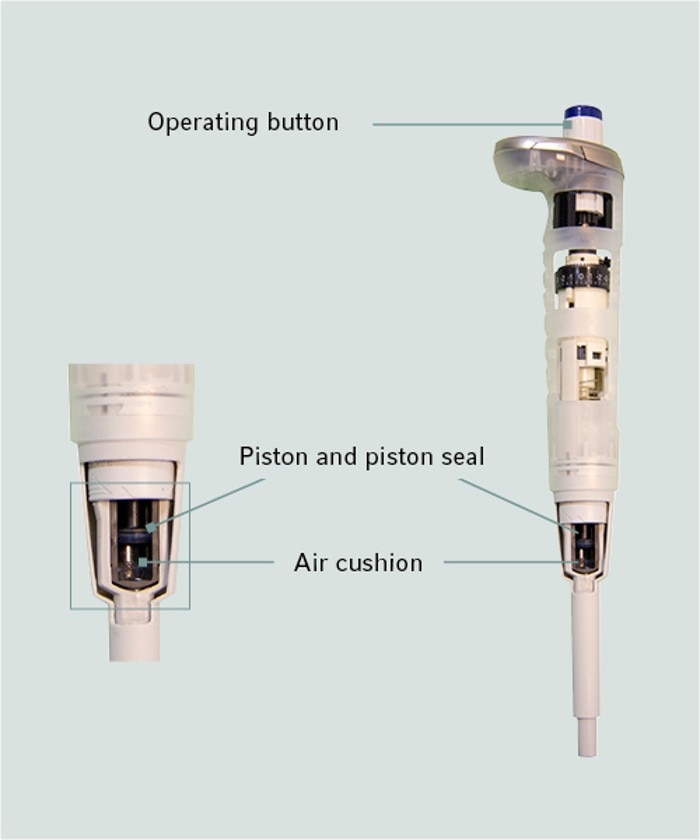
11. What is an air-cushion pipette?
An air-cushion pipette is the most known pipette type. It is used together with pipet tips. Inside the pipette and the pipet tip is air of the surrounding area, the so called air-cushion. This air is moved downwards when pressing the operation button and the sample liquid is aspirated due to the partial vaccuum inside the pipette. The sample liquid is then dispensed by the air pressure when the operation button is pressed down again. The blow-out at the end of a pipetting event is a little amount of extra air that is blown out to deliver all liquid out of the pipet tip.
12. What does positive displacement system mean?
A positive displacement system is mainly used for dispensers, but also some pipettes exist that work according to this principle. Since the instruments themselves do not have a piston, a special tip with an integrated piston is needed to complete the system. When the tip is inserted into the instrument the piston is coupled and gets pulled upwards during usage. The sample liquid is aspirated directly without any air between the tip's piston and the liquid. With this system some difficult liquids like glycerol, ethanol, blood or cell culture medium can be easily aspirated and dispensed.
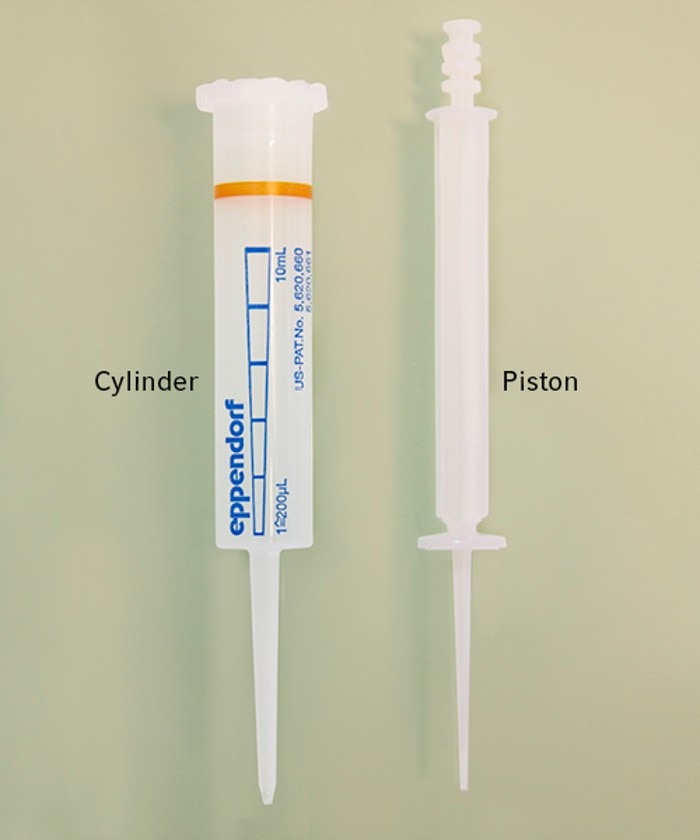
13. What is reverse pipetting?
Read more
Read less
Videos not loading, because cookies have been rejected. Change your

14. When should I use a positive displacement system?
A positive displacement system can be used for any type of sample. But it is recommended especially for viscous, volatile, dense, detergent- and protein-containing liquids. Since it lacks the air-cushion, more precise results can be achieved than with classic pipettes. Often positive displacement systems are so called dispensers. So when working with plates it is advantageous to use a dispenser. With this instrument the whole plate can be filled with only one aspiration, but multiple dispensing steps.

Related files

15. I have noticed that more solution is used for reverse pipetting than for normal pipetting. What is the reason for this?
Reverse pipetting means uptake of liquid including the blow-out volume and dispensing of liquid without blow-out volume. Reverse pipetting is therefore recommended to reduce the error, resulting in more volume being aspirated than necessary. The excess aspirated solution remains in the tip and is discarded.
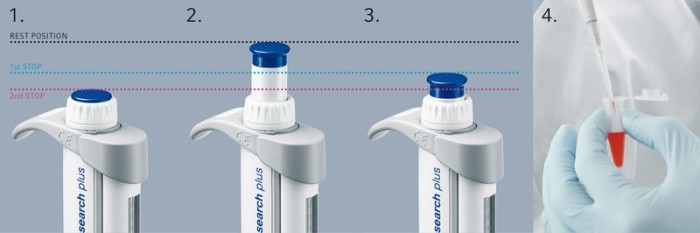
Related files

16. Should the pipette tip be wetted with liquid before pipetting volumes smaller than 10 µL?
Regardless of the volume, the air-cushion should be wetted by multiple filling and emptying of the tip before dispensing. This saturates the air cushion and leads to more accurate and reproducible results.
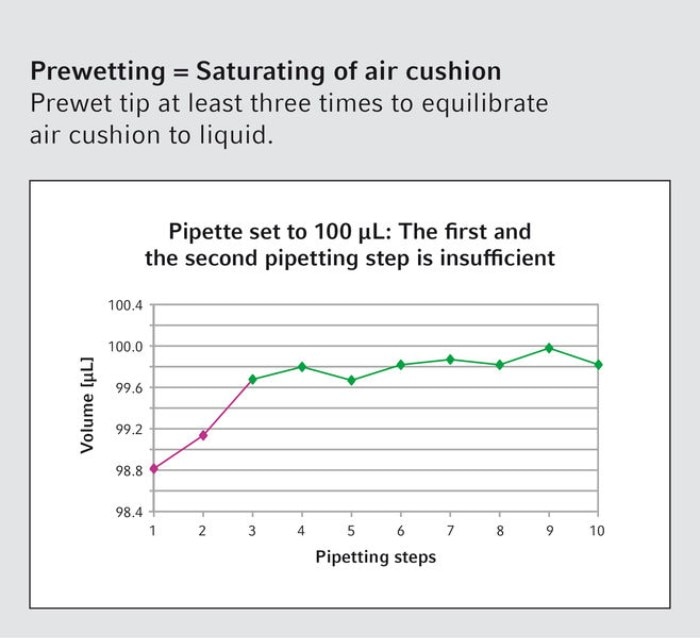
17. What is the recommendation regarding the handling of viscous liquids?
For viscous liquids we recommend positive-displacement instruments like Biomaster, Multipette and Varipette or an air-cushion pipette (Research plus, Reference 2) in combination with reverse pipetting technique (see userguide 20 and 21 for details).
Eppendorf solutions
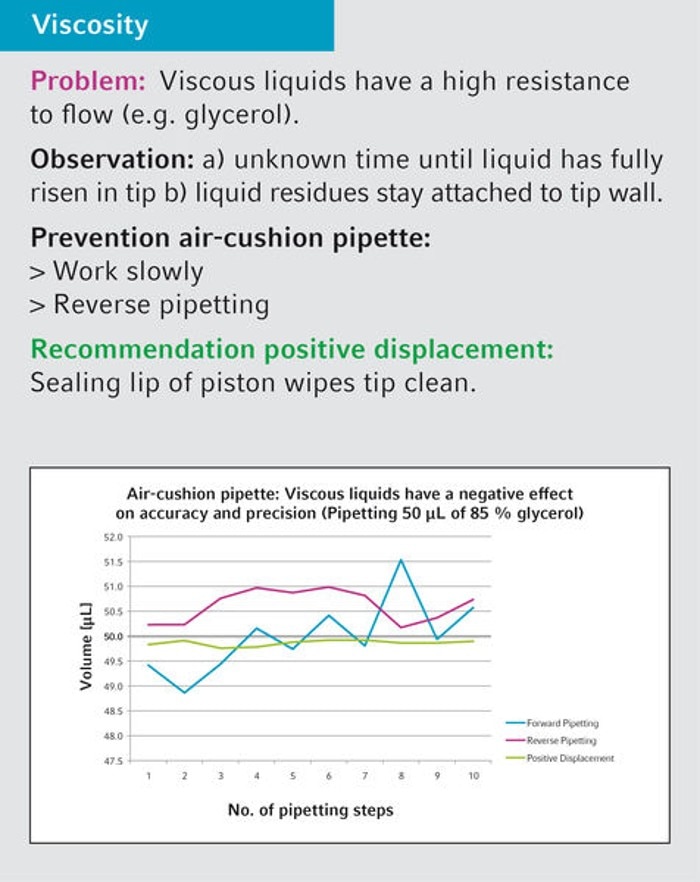
18. Which pipette type can be used to pipette 40 % polyethylene glycol 8000 (PEG)?
Polyethylene glycol is very viscous. Therefore, instruments with positive displacement principle can be used. These systems have an integrated piston in the tip and do not have an air-cushion. Thereby, they are capable of dealing with problematic liquids.
Eppendorf solutions

Related files

19. What is the best way to pipette chloroform?
For chloroform we recommend positive-displacement instruments or an air-cushion pipette in combination with pre-wetting and reverse pipetting technique (see userguide 20 and 21 for details or download the poster "Pipetting techniques" ).
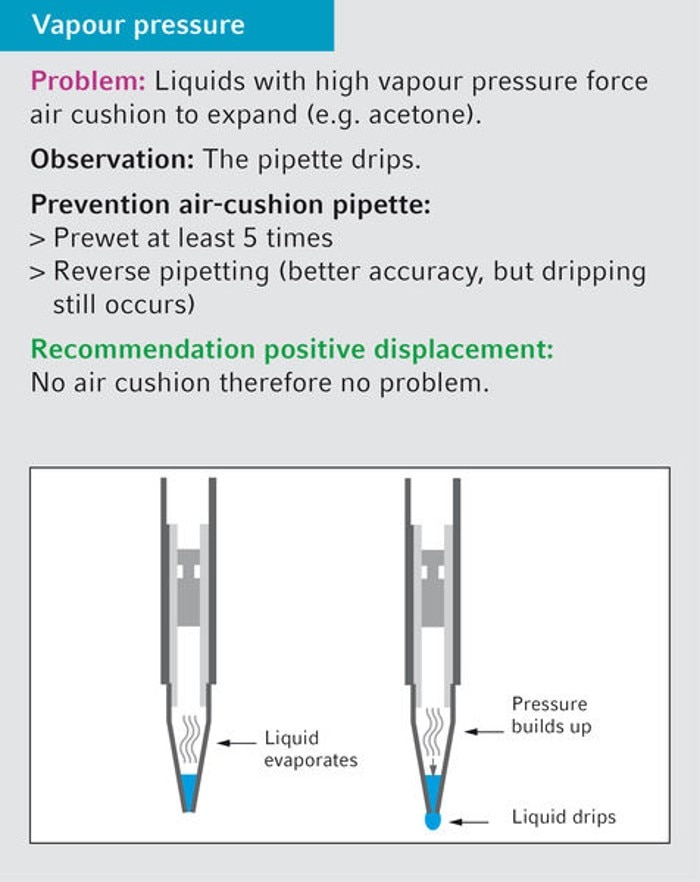
20. What is the best way to pipette diethyl ether?
Diethyl ether has such a high vapor pressure that it would leak out of the tip immediately using an air-cushion pipette. For this reason, it can only be pipetted using a positive-displacement pipette.

21. What should be considered when pipetting methanol with air-cushion pipettes?
Due to the increased vapour pressure of methanol we recommend to pre-wet at least 3 times and perform reverse pipetting technique.

Related files

22. What is the best pipette for pipetting a 99 % solution of n-amyl alcohol?
Read more
Read less
Related files

23. What is the best way to dispense organic solvent?
Read more
Read less
24. I would like to pipette dichloromethane and n-hexanol very precisely. Can I use air-cushion pipettes for this application?
Both dichloromethane and n-hexanol have a very high vapor pressure. Using a normal air-cushion pipette would lead to dripping of the liquid and inaccurate volume delivery. For that reason positive displacement instruments should be used.

25. How can radioactive contamination be removed from pipettes?
Following contamination of pipettes with radioactive liquids, we recommend putting the contaminated parts in a complex-forming liquid or special cleaning solution. After that, rinse very thoroughly with distilled water and let dry. Grease piston lightly afterwards.

26. I very frequently work with different chemicals. Can you tell me from which point I need to adjust my pipettes?
An adjustment of air-cushion pipettes is required for solutions with a density > 1.2 mg/µL. Note that variable manual pipettes turn into "fixed-volume pipettes" when being adjusted. They can only be adjusted for one volume and not across the entire volume range. Sophisticated manual pipettes have a user adjustment for temporary adjustment purposes. This user adjustment can easily be set back to manufacturer settings without the need for recalibration.
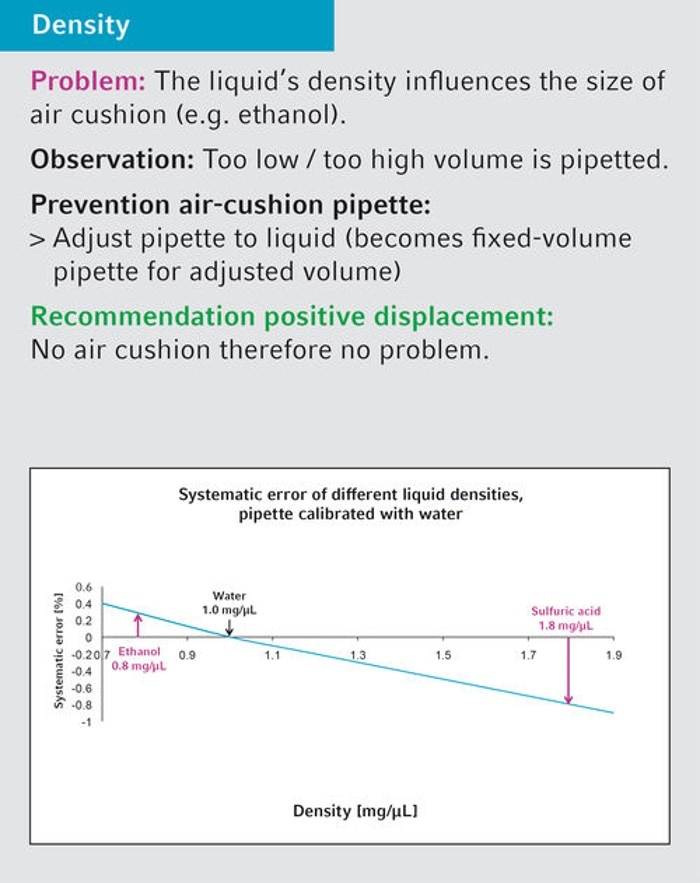
Related files

27. Is it necessary to re-grease the piston of Eppendorf pipettes after autoclaving?
Pipette Cleaning Procedures
1. Disassemble Iower part
2. Rinse / immerse with suitable cleaning fluid (Table 1)
3. Rinse thoroughly with demineralized water
4. Let all parts dry (max. 60 °C or air dry)
5. Lubricate piston or cylinder if necessary1
6. Assemble pipette2
Read more
Read less
| Contaminant | Cleaning fluid |
| Aqueous solutions | Demineralized water |
| Organic solvents | Mild lab detergent |
| Infectious liquids and cell cultures | 4 70% ethanol/isopropanol |
| Nucleic acids | 4 DNA / RNA decontamination agent (follow manufacturer's instruction) |
| Proteins | Mild lab detergent |
| Radioactive substances | Radioactive cleaning fluid |
Read more
Read less
2 After all cleaning procedures, the pipette can be autoclaved as a whole unit at 121 °C, 1 bar overpressure, 20 min. Let all parts dry and cool to room temperature before use.
3 For further information on chemical compability please refer to Chemical Resistance Guide found on the website.
4 Ultra-violet (UV) irradiation is a general surface decontamination and does not remove the contaminant.
Note: Do not use any additional disinfectants, decontamination agents or sodium hypochlorite during autoclaving or UV irradiation.
Read more
Read less
28. Do I need to disassemble the Eppendorf Research® plus before autoclaving?
Pipette Cleaning Procedures
1. Disassemble Iower part
2. Rinse / immerse with suitable cleaning fluid (Table 1)
3. Rinse thoroughly with demineralized water
4. Let all parts dry (max. 60 °C or air dry)
5. Lubricate piston or cylinder if necessary1
6. Assemble pipette2
Read more
Read less
| Contaminant | Cleaning fluid |
| Aqueous solutions | Demineralized water |
| Organic solvents | Mild lab detergent |
| Infectious liquids and cell cultures | 4 70% ethanol/isopropanol |
| Nucleic acids | 4 DNA / RNA decontamination agent (follow manufacturer's instruction) |
| Proteins | Mild lab detergent |
| Radioactive substances | Radioactive cleaning fluid |
Read more
Read less
2 After all cleaning procedures, the pipette can be autoclaved as a whole unit at 121 °C, 1 bar overpressure, 20 min. Let all parts dry and cool to room temperature before use.
3 For further information on chemical compability please refer to Chemical Resistance Guide found on the website.
4 Ultra-violet (UV) irradiation is a general surface decontamination and does not remove the contaminant.
Note: Do not use any additional disinfectants, decontamination agents or sodium hypochlorite during autoclaving or UV irradiation.
Read more
Read less
29. Will water vapor enter the pipette while it is being autoclaved, causing water vapor to bead up on the inside of the numerical volume setting display?
Pipette Cleaning Procedures
1. Disassemble Iower part
2. Rinse / immerse with suitable cleaning fluid (Table 1)
3. Rinse thoroughly with demineralized water
4. Let all parts dry (max. 60 °C or air dry)
5. Lubricate piston or cylinder if necessary1
6. Assemble pipette2
Read more
Read less
| Contaminant | Cleaning fluid |
| Aqueous solutions | Demineralized water |
| Organic solvents | Mild lab detergent |
| Infectious liquids and cell cultures | 4 70% ethanol/isopropanol |
| Nucleic acids | 4 DNA / RNA decontamination agent (follow manufacturer's instruction) |
| Proteins | Mild lab detergent |
| Radioactive substances | Radioactive cleaning fluid |
Read more
Read less
2 After all cleaning procedures, the pipette can be autoclaved as a whole unit at 121 °C, 1 bar overpressure, 20 min. Let all parts dry and cool to room temperature before use.
3 For further information on chemical compability please refer to Chemical Resistance Guide found on the website.
4 Ultra-violet (UV) irradiation is a general surface decontamination and does not remove the contaminant.
Note: Do not use any additional disinfectants, decontamination agents or sodium hypochlorite during autoclaving or UV irradiation.
Read more
Read less
30. How should contaminations on the outside of pipettes be removed?
Pipette Cleaning Procedures
1. Disassemble Iower part
2. Rinse / immerse with suitable cleaning fluid (Table 1)
3. Rinse thoroughly with demineralized water
4. Let all parts dry (max. 60 °C or air dry)
5. Lubricate piston or cylinder if necessary1
6. Assemble pipette2
Read more
Read less
| Contaminant | Cleaning fluid |
| Aqueous solutions | Demineralized water |
| Organic solvents | Mild lab detergent |
| Infectious liquids and cell cultures | 4 70% ethanol/isopropanol |
| Nucleic acids | 4 DNA / RNA decontamination agent (follow manufacturer's instruction) |
| Proteins | Mild lab detergent |
| Radioactive substances | Radioactive cleaning fluid |
Read more
Read less
2 After all cleaning procedures, the pipette can be autoclaved as a whole unit at 121 °C, 1 bar overpressure, 20 min. Let all parts dry and cool to room temperature before use.
3 For further information on chemical compability please refer to Chemical Resistance Guide found on the website.
4 Ultra-violet (UV) irradiation is a general surface decontamination and does not remove the contaminant.
Note: Do not use any additional disinfectants, decontamination agents or sodium hypochlorite during autoclaving or UV irradiation.
Read more
Read less
31. How should disinfection of a pipette be performed?
Pipette Cleaning Procedures
1. Disassemble Iower part
2. Rinse / immerse with suitable cleaning fluid (Table 1)
3. Rinse thoroughly with demineralized water
4. Let all parts dry (max. 60 °C or air dry)
5. Lubricate piston or cylinder if necessary1
6. Assemble pipette2
Read more
Read less
| Contaminant | Cleaning fluid |
| Aqueous solutions | Demineralized water |
| Organic solvents | Mild lab detergent |
| Infectious liquids and cell cultures | 4 70% ethanol/isopropanol |
| Nucleic acids | 4 DNA / RNA decontamination agent (follow manufacturer's instruction) |
| Proteins | Mild lab detergent |
| Radioactive substances | Radioactive cleaning fluid |
Read more
Read less
2 After all cleaning procedures, the pipette can be autoclaved as a whole unit at 121 °C, 1 bar overpressure, 20 min. Let all parts dry and cool to room temperature before use.
3 For further information on chemical compability please refer to Chemical Resistance Guide found on the website.
4 Ultra-violet (UV) irradiation is a general surface decontamination and does not remove the contaminant.
Note: Do not use any additional disinfectants, decontamination agents or sodium hypochlorite during autoclaving or UV irradiation.
Read more
Read less
32. What do I have to observe regarding maintenance and cleaning of pipettes?
Pipette Cleaning Procedures
1. Disassemble Iower part
2. Rinse / immerse with suitable cleaning fluid (Table 1)
3. Rinse thoroughly with demineralized water
4. Let all parts dry (max. 60 °C or air dry)
5. Lubricate piston or cylinder if necessary1
6. Assemble pipette2
Read more
Read less
| Contaminant | Cleaning fluid |
| Aqueous solutions | Demineralized water |
| Organic solvents | Mild lab detergent |
| Infectious liquids and cell cultures | 4 70% ethanol/isopropanol |
| Nucleic acids | 4 DNA / RNA decontamination agent (follow manufacturer's instruction) |
| Proteins | Mild lab detergent |
| Radioactive substances | Radioactive cleaning fluid |
Read more
Read less
2 After all cleaning procedures, the pipette can be autoclaved as a whole unit at 121 °C, 1 bar overpressure, 20 min. Let all parts dry and cool to room temperature before use.
3 For further information on chemical compability please refer to Chemical Resistance Guide found on the website.
4 Ultra-violet (UV) irradiation is a general surface decontamination and does not remove the contaminant.
Note: Do not use any additional disinfectants, decontamination agents or sodium hypochlorite during autoclaving or UV irradiation.
Read more
Read less
33. What is an RFID chip used for?
The RFID chip is used to clearly identify an instrument. Identification data, e.g. serial number or results of quality control test of manufacturer, are stored on the RFID chip. These data can not be changed or deleted.
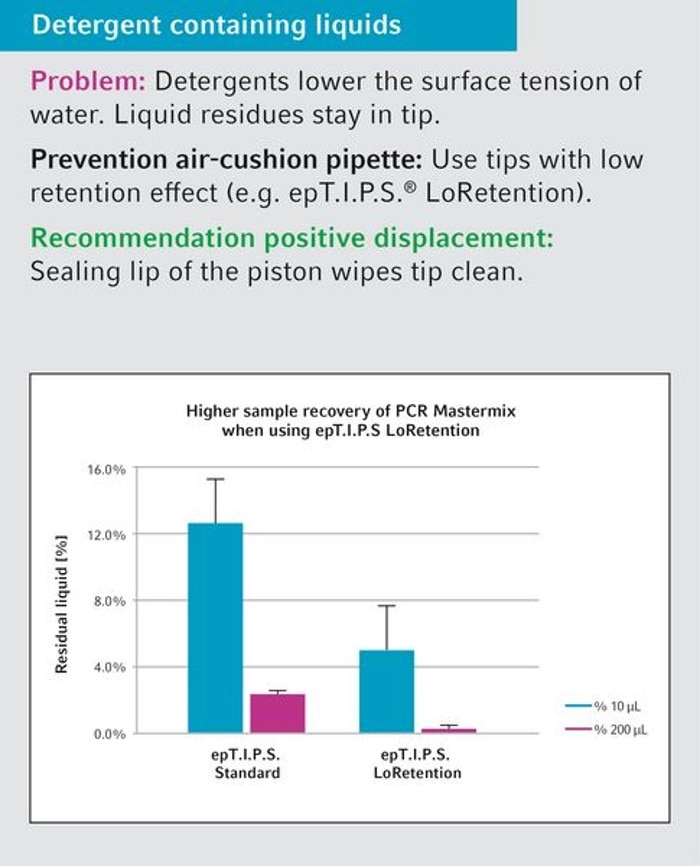
34. Under which conditions will the use of low retention tips be advantageous?
Tips with low retention properties will grant an advantage especially during pipetting of solutions containing detergents, such as PCR master mixes, enzyme solutions and many buffers. These tips are characterized by significantly improved flow performance, thus achieving more economic use of expensive reagents, while valuable sample material is used effectively. Furthermore, reproducibility is improved, since wetting of the tip is dependent on both user and handling (for example: pipetting speed) and can therefore vary considerably.
35. Will foam formation, which is observed during pipetting of detergent containing solutions, be reduced when low retention tips are used?
36. Does the self-sealing mechanism of a blocking filter work with all kinds of liquids?
The self-sealing mechanism works best with aqueous solutions, most acids and bases. Strong acids and bases might not be blocked properly and increase the risk of damaging the pipette if accidental over-pipetting occurs. For very aggressive solutions we recommend positive displacement systems where the sample is secured in the tip without any contact to the liquid handling instrument.