MENU
IE | EUR
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- DataHow Symposium 2025
- Cell 2025
- LabDays 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- DataHow Symposium 2025
- Cell 2025
- LabDays 2025
- ASIA LABEX: The Lab Show 2025
IE | EUR
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- IVD Products
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
No results found
Search Suggestions
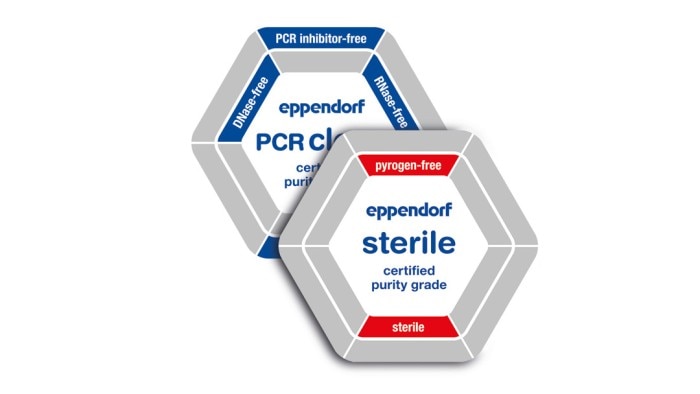
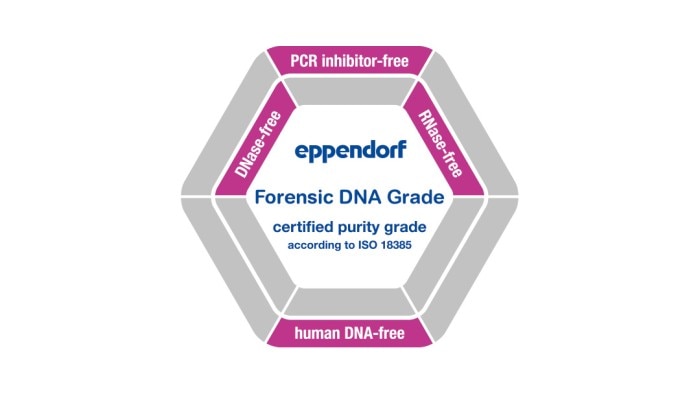
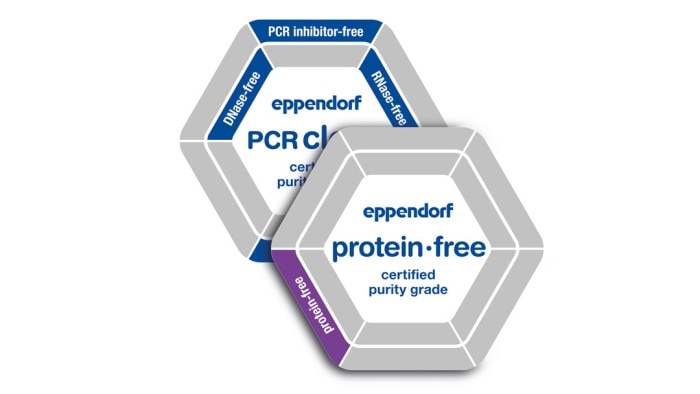
*The suffix "-free" in connection with the purity classes mean that the test showed conformity within the detection limits (see lot-specific certificate). Learn more
Read more
Read less
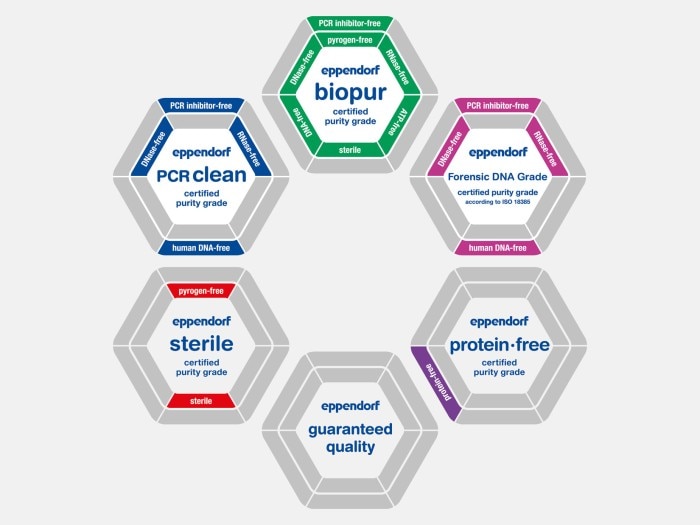
Which are the right purity grades for my applications? Here you will find an overview.
Read more
Read less
Eppendorf consumables stand for the highest quality and purity. This is achieved by the following:
- Manufacturing using the purest raw materials
- Fully automatic production in clean room conditions
- Quality and functional checks of all lots
- Continuous quality assurance throughout the entire production process – from the initial material to the finished product
Read more
Read less
With the strictest control criteria, internally and externally monitored, we guarantee the consistently high quality of our products – lot by lot.
Our consumables are available in a variety of purity grades. In addition to our internal process controls, products with the purity grades Sterile, Protein-free, PCR clean, Forensic Grade or Biopur are tested for biological contamination by an accredited external lab. This allows us to ensure we consistently meet our customer‘s high demands when it comes to the high quality and purity of our products. As a special service, we provide the corresponding lot-specific certificate of analysis (CoA) online for each batch delivered.
Our consumables are available in a variety of purity grades. In addition to our internal process controls, products with the purity grades Sterile, Protein-free, PCR clean, Forensic Grade or Biopur are tested for biological contamination by an accredited external lab. This allows us to ensure we consistently meet our customer‘s high demands when it comes to the high quality and purity of our products. As a special service, we provide the corresponding lot-specific certificate of analysis (CoA) online for each batch delivered.
Read more
Read less
Shelf life of Eppendorf Consumables according to the purity levels
| Minimum shelf life | |
| Eppendorf Quality | 8 years |
| Purity Grades: | |
| Sterile | 5 years |
| PCR clean | 5 years |
| Biopur | 5 years |
| Forensic DNA Grade | 5 years |
| PCR clean+ sterile | 5 years |
| PCR-clean+ Protein-free | 5 years |
Read more
Read less
The shelf life can only be guaranteed for sealed and adequately stored items. The storage conditions can also be found in the operating instructions in the section "Technical data".
The exact expiry date is indicated on the product label in the following format: YYYY-MM-DD, where the exact expiry date is always the 28th of the month. This format follows FDA recommendations for the Unique Device Identification (UDI) system.
The shelf life of 8 years of our consumables in "Eppendorf Quality" relates to the technical material properties of our products. The limited shelf life of 5 years for the higher purity grades (e.g., sterility) refers to the products’ purity, as it may be more strongly influenced by packaging and storage conditions than the material properties.
The exact expiry date is indicated on the product label in the following format: YYYY-MM-DD, where the exact expiry date is always the 28th of the month. This format follows FDA recommendations for the Unique Device Identification (UDI) system.
The shelf life of 8 years of our consumables in "Eppendorf Quality" relates to the technical material properties of our products. The limited shelf life of 5 years for the higher purity grades (e.g., sterility) refers to the products’ purity, as it may be more strongly influenced by packaging and storage conditions than the material properties.
Read more
Read less
 |  |  |  |  |  | |
| Methods (Examples) | ||||||
| Applications requiring high general quality, but no checked special purities | ||||||
| Bacteria and yeast culture | ||||||
| Cell and tissue culture | ■ ■ | |||||
| Isolation and storage of DNA | ■ ■ | ■ | ■ ■ | ■ | ||
| Isolation and storage of RNA | ■ | ■ | ■ | ■ ■ | ||
| DNA analysis (PCR, restriction analysis, hybridization, sequencing , NGS) | ■ ■ | ■ | ■ ■ | ■ | ||
| Mitochondrial DNA analysis | ■ ■ | ■ ■ | ||||
| Bacterial DNA analysis | ■ ■ | |||||
| RNA analysis | ■ | ■ ■ |
Read more
Read less