MENU
MY | MYR
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
MY | MYR
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.
No results found
Search Suggestions

Finalizing the Run with Amplicons in Your Negative Control?
Lab Academy
- Molecular Biology
- Amplification & PCR
- Contamination
- PCR Cyclers
- Essay
The major topic for PCR labs is contamination: The big “C-threat” and how you can reduce the risk of it to a minimum. Best-practices, logic approaches, discussions with other lab teams – there are many ways to improve the process in your own lab.
Contamination seems to be the bane of Life Science. There is no field of interest in biology where you can simply ignore discussions and taking action steps against contamination. At times, it is as simple as being mindful of everything we do and use – such as proper cleaning, handling, clothing, and using good consumables. However, for pioneers who set up labs from scratch – be it in academia or a new start-up company – good lab practice starts from designing proper workspaces for people to work and clean every day.
This is especially true for workflows that involve amplification such as PCR, since anything small – including miniscule amount of contaminated source – will get amplified a million times. No matter how good you are at everyday lab practices, it only takes one instance of cross-contamination to mess everything up, which can subsequently lead to major waste of resources to find, solve and eliminate future incidents. Hence, it is always a good idea to take preventive step from the beginning. One way to minimize cross-contamination in PCR from early on is to be able to dedicate different work functions to different areas. In other words, you perform each main function in a separate room. This helps to minimize aerosol contamination from one place to another as you move along the experimental workflow.
This is especially true for workflows that involve amplification such as PCR, since anything small – including miniscule amount of contaminated source – will get amplified a million times. No matter how good you are at everyday lab practices, it only takes one instance of cross-contamination to mess everything up, which can subsequently lead to major waste of resources to find, solve and eliminate future incidents. Hence, it is always a good idea to take preventive step from the beginning. One way to minimize cross-contamination in PCR from early on is to be able to dedicate different work functions to different areas. In other words, you perform each main function in a separate room. This helps to minimize aerosol contamination from one place to another as you move along the experimental workflow.
Read more
Read less
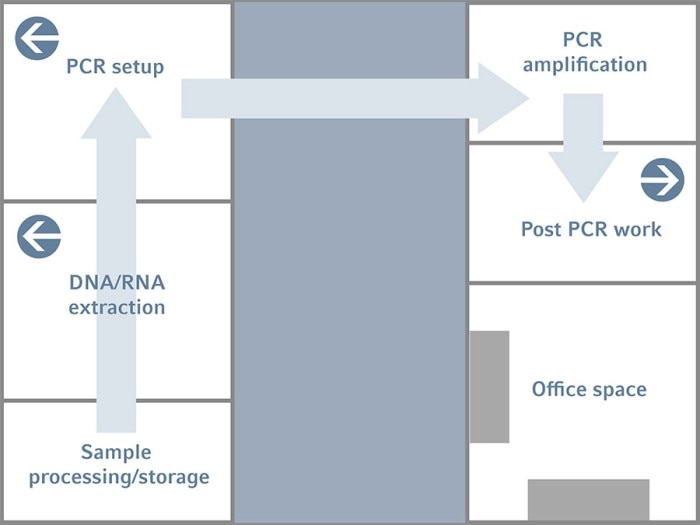
Figure 1: Example of a lab design with separated rooms along the linear PCR workflow to reduce the risk of cross-contamination within the process
In the simplified diagram of the lab design shown in Figure 1, you will notice that each of the main task in the PCR workflow is dedicated to one room and that the sample goes in an unidirectional flow. Starting with the sample processing/storage room, this is where you keep or perform a certain processing step on the raw sample that you have received. For example, in a GMO lab, here is where the foodstuff is processed into finer components before it is used for DNA extraction. The raw sample is then transferred to the next room – the Sample Preparation room for subsequent DNA/RNA extraction.
The extracted nucleic acid samples are then brought to the next room – the PCR Setup room. Here is where we put all the reaction components into a tube to form a PCR reaction mix. Once this reaction mix is setup, it will be transferred to another room called the PCR Amplification Room. This is the room where thermal cyclers are placed. The only thing that you should do in this room is to put the reaction tubes into the thermal cyclers and let them run. Nothing else is performed.
However, depending on the exact workflow and resources, it is usual to see different thermal cyclers dedicated for pre-PCR (e.g. cDNA synthesis), PCR and even post-PCR (sequencing). Sometimes, these cyclers are even separately placed in different rooms. In such cases, the PCR setup and PCR amplification rooms are usually combined for each of these prePCR, PCR, and post-PCR steps. The main point is on minimizing cross-contamination from one part of the workflow to the next. It is also easier to control, troubleshoot and eliminate any contamination that does occur this way.
The Electrophoresis room is the last room where you check the results of the reaction. The end product – that is, the PCR product – will be left here and should not be brought out of the room unless you intend to do some further experiments with the products.
To further avoid cross-contamination of aerosols from one room to another, the PCR setup room will have a slightly positive pressure so that there is a constant flow of air from the room to the outside environment. This will prevent the entry of aerosols. On the other hand, the Electrophoresis room will have a slightly negative pressure and this will draw a constant stream of air into the room, making sure no aerosols can escape the room. At the same time, the red dots in these rooms represent each room has its own exhaust system. This is installed in order to channel any aerosols out of each room individually to minimize crosscontamination. The air-conditioning system in each room should also be decentralized.
Of course, not everybody can afford the luxury of a huge lab with multiple rooms. With smaller labs, you make the best out of what you have by rearranging where and how each main function should be performed (Figure 2). In this setup, there should be a separate bench for each critical task along the PCR workflow – DNA extraction, RNA extraction, and PCR setup. Additionally, the electrophoresis area should at the very least be isolated by proper partition.
The extracted nucleic acid samples are then brought to the next room – the PCR Setup room. Here is where we put all the reaction components into a tube to form a PCR reaction mix. Once this reaction mix is setup, it will be transferred to another room called the PCR Amplification Room. This is the room where thermal cyclers are placed. The only thing that you should do in this room is to put the reaction tubes into the thermal cyclers and let them run. Nothing else is performed.
However, depending on the exact workflow and resources, it is usual to see different thermal cyclers dedicated for pre-PCR (e.g. cDNA synthesis), PCR and even post-PCR (sequencing). Sometimes, these cyclers are even separately placed in different rooms. In such cases, the PCR setup and PCR amplification rooms are usually combined for each of these prePCR, PCR, and post-PCR steps. The main point is on minimizing cross-contamination from one part of the workflow to the next. It is also easier to control, troubleshoot and eliminate any contamination that does occur this way.
The Electrophoresis room is the last room where you check the results of the reaction. The end product – that is, the PCR product – will be left here and should not be brought out of the room unless you intend to do some further experiments with the products.
To further avoid cross-contamination of aerosols from one room to another, the PCR setup room will have a slightly positive pressure so that there is a constant flow of air from the room to the outside environment. This will prevent the entry of aerosols. On the other hand, the Electrophoresis room will have a slightly negative pressure and this will draw a constant stream of air into the room, making sure no aerosols can escape the room. At the same time, the red dots in these rooms represent each room has its own exhaust system. This is installed in order to channel any aerosols out of each room individually to minimize crosscontamination. The air-conditioning system in each room should also be decentralized.
Of course, not everybody can afford the luxury of a huge lab with multiple rooms. With smaller labs, you make the best out of what you have by rearranging where and how each main function should be performed (Figure 2). In this setup, there should be a separate bench for each critical task along the PCR workflow – DNA extraction, RNA extraction, and PCR setup. Additionally, the electrophoresis area should at the very least be isolated by proper partition.
Read more
Read less
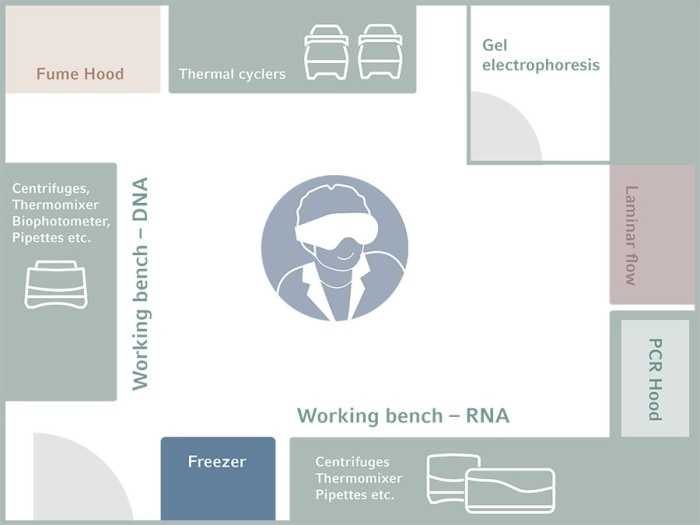
Figure 2: Example of lab design for PCR in a limited space to reduce the risk of contamination
Naturally, things are not rigidly set in stone. Depending on the amount of space you have at your disposal as well as the total experimental workflow involved including the pre- and post- PCR parts, a mixture of these lab designs can be implemented. For example, in Genetek Biopharma® , an Eppendorf customer whom we visited and interviewed, they have similarly put much effort into the logical design of a lab suited for their purposes in ensuring smooth workflow as well as securing the quality of their diagnostic kits during development phase.
“We started with a single lab for PCR and we separated the work areas, but now we are planning to expand into a separate new lab – separating our PCR setup (room) from the production room” said Mr. Grail.
According to Genetek Biopharma’s expansion plan, the PCR mix, DNA controls, and DNA samples are prepared in the pre-PCR room. In addition to following good lab practices such as cleaning and sterilization of work benches with surface decontaminant prior to kit reagent preparation, the separation of work areas into pre-PCR and post-PCR rooms provide another layer of security in safeguarding kit quality. After proper preparation, a transporting rack is used to transfer the reaction mixture from the pre-PCR room to the post-PCR room. This helps to minimize the flow of objects from one room to the other as well as serves to prevent contamination of the components in the pre-PCR room with PCR product.
In the post-PCR room, PCR is performed, agarose gel is prepared, and gel electrophoresis is performed to check whether the target DNA sequences have been amplified. The Genetic Analyzer 3500XL is used to perform fragment analysis and quality control of the PCR product(s). If quality control is passed, the kit reagents are aliquoted and stored at -20°C until dispatch. If spectral calibration is necessary, a matrix standard solution will also be included. Depending on the kit, an allelic ladder will also be added.
“We started with a single lab for PCR and we separated the work areas, but now we are planning to expand into a separate new lab – separating our PCR setup (room) from the production room” said Mr. Grail.
According to Genetek Biopharma’s expansion plan, the PCR mix, DNA controls, and DNA samples are prepared in the pre-PCR room. In addition to following good lab practices such as cleaning and sterilization of work benches with surface decontaminant prior to kit reagent preparation, the separation of work areas into pre-PCR and post-PCR rooms provide another layer of security in safeguarding kit quality. After proper preparation, a transporting rack is used to transfer the reaction mixture from the pre-PCR room to the post-PCR room. This helps to minimize the flow of objects from one room to the other as well as serves to prevent contamination of the components in the pre-PCR room with PCR product.
In the post-PCR room, PCR is performed, agarose gel is prepared, and gel electrophoresis is performed to check whether the target DNA sequences have been amplified. The Genetic Analyzer 3500XL is used to perform fragment analysis and quality control of the PCR product(s). If quality control is passed, the kit reagents are aliquoted and stored at -20°C until dispatch. If spectral calibration is necessary, a matrix standard solution will also be included. Depending on the kit, an allelic ladder will also be added.
Read more
Read less

Figure 3: Genetek Biopharma’s Post-PCR room: DNA amplification is performed in the thermal cyclers and gel electrophoresis is performed at a separate corner in this room (not in the photo). The centrifuges are used during the purification process of the pooled size standard and matrix standard fragments and fragment analysis is performed with the genetic analyzer for quality control.
In conclusion, physical separation of workspaces is indispensably beneficial for minimizing risk of cross-contamination in PCR workflow. Proper lab design with such separation can be implemented according to the amount of space available at your disposal, including the use of PCR workstation cabinets.
Genetek Biopharma® is a registered trademark of Genetek Biopharma GmbH, Germany. All rights reserved including graphics and images. Copyright © 2019 Eppendorf AG
Genetek Biopharma® is a registered trademark of Genetek Biopharma GmbH, Germany. All rights reserved including graphics and images. Copyright © 2019 Eppendorf AG
Read more
Read less
