
PCR Turns 40! From Lab Curiosity to Revolutionary Tool - A Look Back and Ahead
Lab Academy
- Molecular Biology
- Microbiology
- PCR Cyclers
- Lab Routine
- Amplification & PCR
- Essay
This year marks the 40th anniversary of PCR - the polymerase chain reaction - a milestone that transformed molecular biology, diagnostics, and many other fields. Developed by Kary Mullis in 1983, PCR has come a long way from its early days of manual heating and cooling to today's fast and automated processes. Join us on a thrilling ride through the history of PCR - the game-changing technique that revolutionized DNA amplification and paved the way for modern biotechnology!
The Birth of PCR: Mullis, Primers, and Polymerases
PCR relies on three key components: a DNA template, two short DNA primers that flank the target sequence, and a heat-stable DNA polymerase enzyme. By repeatedly heating and cooling the mixture, the primers bind to the template, the polymerase extends the new DNA strand, and the cycle starts anew, doubling the amount of DNA each time. In just a few minutes, PCR can amplify millions or billions of copies of a specific DNA segment.
Originally, PCR was developed for the detection of mutations in the HBB gene that causes sickle cell anemia. Mullis noticed that the Sanger sequencing method yielded only weak signals and identified the reason: insufficient DNA concentration! Adding a denaturation step to split each dsDNA molecule into two ssDNAs, and a reverse primer to define the total amplicon length, resulted in the amplification of the DNA copies by two (1,2,3). For the PCR breakthrough, Mullis was awarded the Noble Prize in 1993.
Read more
Read less
Kary Banks Mullis (1944-2019) was an American biochemist who received the Nobel Prize in Chemistry in 1993 for his invention of the PCR. Mullis earned a Ph.D. in biochemistry from the University of California, Berkeley, and worked as a researcher at several biotech companies, including Cetus Corporation, where he developed PCR in 1983. In addition to his scientific achievements, Mullis was known for his unconventional personality, including his interests in surfing, guitar, astrology, and psychedelic drugs.

The Early PCR Technology: A Striking Game-Changer
The first PCR experiments were done manually, using simple tap water baths or block heaters for heating and cooling. The entire process could take several hours, and samples needed to be secured by mineral oil. However, even this "primitive" PCR was a game-changer, as it enabled researchers to amplify DNA from various sources, and to analyze them in unprecedented detail.
In 1988, the first commercial automated PCR cycler came to market. PCR thermal cyclers quickly became a standard in laboratories around the world and helped make PCR technology even more accessible.
Read more
Read less

PCR Comes of Age: Thermal Cycler Revolutionize the Market
After the invention of a method for monitoring PCR kinetics in real time, PCR techniques became fully quantitative. This led to the development of Real-Time PCR (or qPCR), which has the ability to detect PCR products in real time and provides rapid and accurate measurements of nucleic acid concentration. Thermal cyclers were designed for qPCR harboring optical systems for monitoring fluorescence during the reaction, like the ABI LS-50 or the Lightcycler from Roche. The incorporation of reverse transcription (RT) before thermal cycling allowed the use of PCR in RNA studies (RT-PCR or qRT-PCR).
In 1997, the first gradient cycler revolutionized the market: the Mastercycler Gradient. The gradient technology developed by Eppendorf for PCR optimization aimed to address the difficulties with manual optimization of the annealing temperature, usually carried out by multiple PCR runs with varying conditions. Imagine, ramp rates of 2 °C/s were super-fast then!
Read more
Read less
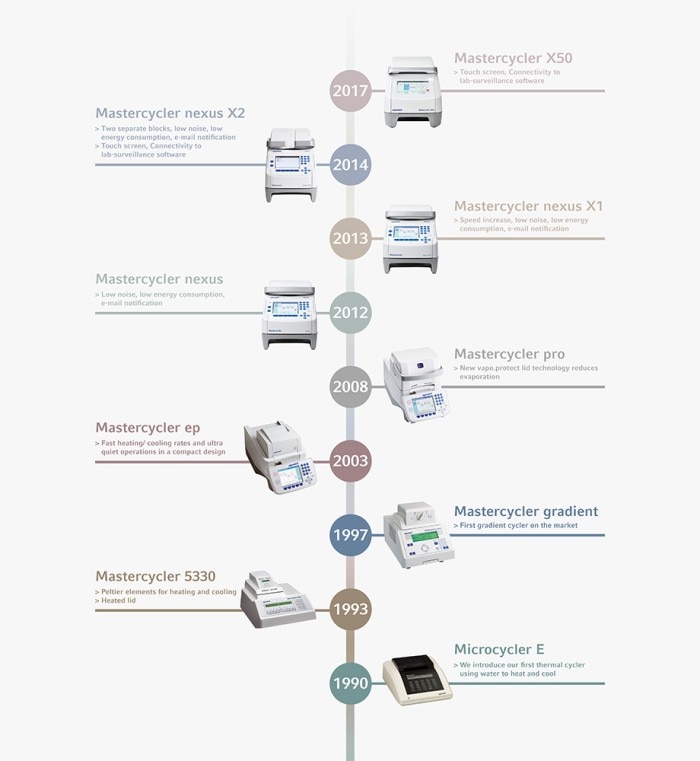
As scientific research advances, there is an increased requirement to reliably gain access to regions of DNA with more complex sequences and to enable specific amplification resulting in high yield for subsequent applications, such as Next Generation Sequencing. For these purposes, it is both important to address this high complexity and the variation between different PCR primers. In 2017, Eppendorf has developed the Mastercycler® X50 with innovative 2D-Gradient technology that combines the optimization of annealing and denaturation temperature – all in a single run. This was the next milestone in PCR optimization.

Read more
Read less
PCR Beyond Limits: Today & Tomorrow
Real-time PCR has revolutionized the 21st century due to its tremendous applications in quantitative genotyping, detecting genetic variations within and between organisms, and early diagnosis of diseases. The combination of real-time PCR with other molecular techniques has made it possible to monitor therapeutic interventions and individual responses to drugs, among other things. Also, qPCR has replaced the time-consuming and tedious slot blot technique for forensic investigations (5).
One of the most promising methods is loop-mediated isothermal amplification (LAMP) , which allows amplification at constant temperatures without a thermal cycler. LAMP has huge potential in applications in point-of-care diagnostics like pathogen detection from minimally processed sample such as the recently developed SARS-CoV-2 test that can be performed at home using only a smartphone and no laboratory equipment (6).
Read more
Read less
Several breakthrough technologies rely on PCR, such as NGS, nanopore sequencing, and syntheticbiology. CRISPR is a revolutionary gene editing tool that relies on a bacterial defense system to cut andpaste DNA with unprecedented precision and ease. CRISPR uses PCR as a crucial step in its workflow, toamplify and prepare the DNA fragments for editing.
Ultimately, PCR is a ubiquitous and versatile tool that is used in almost every field of biology andmedicine, from forensics to food safety, from cancer diagnosis to infectious disease surveillance.
Read more
Read less
Cheers to PCR!
Milestones in the history of PCR:
1952: Rosalind Franklin produces photographs of DNA fibers using X-ray diffraction1953: Watson and Crick discover the double helix structure of DNA
1976: Taq Polymerase is isolated from Thermus aquaticus
1983: Kary Mullis develops the PCR technique
1985: The first paper describing the PCR technique is published in the journal Science by Saiki et al.
1986: PCR is used for the first time in a criminal case, to identify a suspect in a murder case
1987: The first commercial PCR machine is introduced, allowing researchers to automate the process
1988: PCR is used to amplify and sequence ancient DNA from a 40,000-year-old Neanderthal specimen
1989: DNA from single sperm cells is amplified
1991: First DNA polymerase with proof-reading activity isolated from Thermococcus litoralis is published
1993: First PCR chip for miniaturizing PCR is developed
1993: Kary Mullis is awarded for the Nobel Prize in Chemistry for the invention of PCR
1994: PCR is used to amplify and detect the first genetically modified organism (GMO), a tomato
1996: Real-time PCR is developed, allowing researchers to monitor the amplification process in real-time
1996: The first cloned mammal was produced - the sheep Dolly
2005: Next Generation Sequencing comes into market, using PCR for the generation of NGS libraries
2007: The first complete human genome is sequenced
2020: PCR is used extensively in the COVID-19 pandemic, for the detection of SARS-CoV-2
2023: PCR celebrates its 40th anniversary!
Literature
1. Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 51(Pt. 1), 263–273 (1986).2. Saiki RK, Scharf S, Faloona F. et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle-cell anemia. Science 230(4732), 1350–1354 (1985)
3. Zhu H, Zhang H, Xu Y, Laššáková S, Korabečná M, Neužil P. PCR past, present and future. Biotechniques. 2020 Oct;69(4):317-325. doi: 10.2144/btn-2020-0057. Epub 2020 Aug 20.
4. https://en.wikipedia.org/wiki/Thermal_cycler
5. Deepak S, Kottapalli K, Rakwal R, Oros G, Rangappa K, Iwahashi H, Masuo Y, Agrawal G. Real-Time PCR: Revolutionizing Detection and Expression Analysis of Genes. Curr Genomics. 2007 Jun;8(4):234-51. doi: 10.2174/138920207781386960. PMID: 18645596; PMCID: PMC2430684.
6. Heithoff, D.M. et al. (2022). Assessment of a Smartphone-Based Loop-Mediated Isothermal Amplification Assay for Detection of SARS-CoV-2 and Influenza Viruses. JAMA Network Open, 5 (1).
Read more
Read less
