MENU
SK | EUR
SK | EUR
No results found
Search Suggestions

Trojan Horse Intrigue: Leaching Fake DNA in Your qPCR
Lab Academy
- Molecular Biology
- Lab Life
- Lab Routine
- Efficiency
- Next Generation Sequencing
- Amplification & PCR
- Photometry
- Reproducibility
- Automation
- Essay
Discover how leachables, like the infamous Trojan Horse, can silently compromise the integrity of your molecular biology techniques like qPCR or NGS. Learn about their negative effects on PCR assays, the reallife impacts observed in studies, and how to mitigate this hidden threat. Don't let leachables sabotage your results - uncover the truth and safeguard your research!
Leachables: The Trojan Horse
Leachables are chemical substances like plasticizers, biocides or slip agents that may emerge from the materials used in the production of consumables, including plastics, adhesives, coatings, or other components. Although transparent wells are usually perceived as a high-quality parameter, the well clarity often correlates with high amounts of clarifying agents utilized during the production process. Just like the Trojan Horse, the sneaky substances can secretly migrate (leach) into the samples or reagents during processing or storage under different conditions, such as temperature, time, or solvent exposure.Trojan Horse effects
Leachables are a concern in molecular biology, diagnostics, and analytical sciences, because they can potentially affect the integrity, purity, or functionality of the samples or reagents and interfere with a broad range of assays:- Nucleic acid quantification, e.g. photometric assays
- Sample isolation
- NGS library preparation
- PCR, qPCR, sequencing
- Enzymatic assays
- Receptor binding assays
- Ligation and cloning reactions
- Amplification assays for downstream analysis workflows
Numerous studies indicate that leachables may adversely affect assay accuracy, reproducibility, reliability, and data validity in terms of false positives and false negatives [1-8] . Leachables may also cause alterations and growth reduction in various cell culture systems [6, 8] . For plate-based assays, the leaching effects are particularly relevant, where a high variability in temperature conditions as well as position-dependent leaching (Fig. 3) can dramatically influence the data validity and reproducibility of a PCR/qPCR assay [4] .
Hot on the trail: Proven real-life examples
Recently, leachable levels were assessed within a comparative analysis of PCR plates from several manufacturers showing that leaching may strongly influence qPCR assays and interfere with both photometric and fluorescence signal quantification leading to poor reproducibility results. Furthermore, a high variability of leaching itself was observed in some plates, which may adversely affect intra- and inter-plate assay reproducibility:1. PCR plates can release considerable amounts of leachables (up to 3.08 μg/mL, Fig. 1)
2. Leachables act like fake nucleic acids because their UV-absorbing property closely mimics the spectrum of nucleic acids (Fig. 1). These UV-active leachables may heavily influence spectrophotometric quantification of nucleic acids, leading to false DNA or RNA readings and interfering with downstream applications.
Read more
Read less
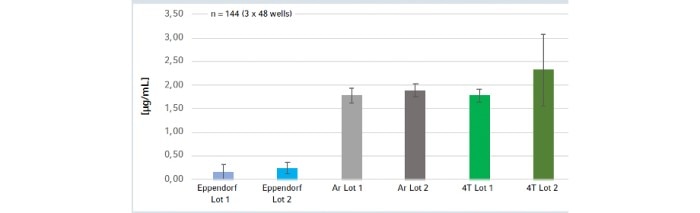
Fig.1: Fake DNA concentration based on UV-absorbing leachables. Plates from different manufacturers and lots were analyzed and compared with Eppendorf twin.tec® PCR plates.
3. Leachables interfere with fluorescence signal quantification (SYBR and CY5 detection channels) in qPCR assays leading to false or unreproducible results (Fig. 2).
Read more
Read less
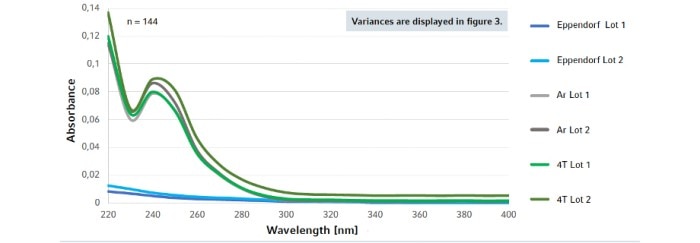
Fig. 2: Absorption spectra of nucleic acid-mimicking leachables. Plates from different manufacturers and lots were analyzed and compared with Eppendorf twin.tec® PCR plates.
4. Leaching within the same plate or between different plates highly varies and may adversely affect intra- and inter-plate assay reproducibility (Fig. 3). A position-dependent leaching may dramatically influence both intra- as well as inter-plate reproducibility and thus data validity of a qPCR assay [4] .
Read more
Read less
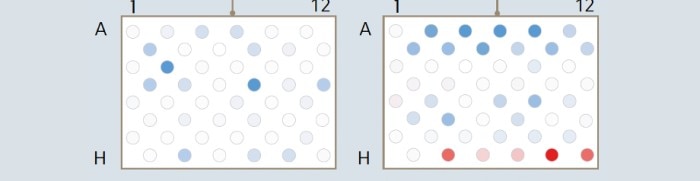
Fig. 3: Potential real-life impact of a fluctuating “blank signal” variance in plates of different lots. It demonstrates how such fluctuation might lead to decreased meaningfulness of reference samples. Displayed are median-centered absorption values in decreasing intensity from red (high signal) to blue (low signal). The signals in lot 2 show a much higher variance across the plate. In the laboratory practice, this might lead to false positive or false negative results.
Noteworthy, Eppendorf PCR plates exhibited the by far lowest levels of leachables and no intra- and inter-plate variability of leaching in this analysis (Fig. 1,2)!
To mitigate the potential impact of leachables, it is important to use high-quality consumables from reputable manufacturers that provide documentation on the materials used, their composition, and potential leachables. It is also advisable to conduct appropriate validation or characterization studies to assess the potential presence and impact of leachables on specific assays or experiments. Additionally, using suitable controls, blanks, or reference materials can help identify and quantify potential leachables in samples or reagents. Significant reduction of such sources of error and applicational risks might be achieved by using high quality plates, such as the Eppendorf PCR plates.
What you need to know
Literature
[1] Lewis LK, Robson M, Vecherkina Y, Ji C, Beall G. Interference with spectrophotometric analysis of nucleic acids and proteins by leaching of chemicals from plastic tubes. Biotechniques 2010; 48(4):297-302. [2] Grzeskowiak R. Extractables and Leachables in Microcentrifuge Tubes – Extensive HPLC/GC/MS Analysis. Eppendorf AG Application Note. 2018: No. 417; www.eppendorf.com
[3] McDonald GR, Hudson AL, Dunn SM, You H, Baker GB, Whittal RM, Martin JW, Jha A, Edmondson DE, Holt A. Bioactive contaminants leach from disposable laboratory plasticware. Science 2008; 322(5903):917
[4] Pfaffl M. Afif M. How to apply the MIQE Guidelines – a visual, interactive and practical qPCR guide! iBook (ISBN-9783000488061)
[5] McDonald GR, Kozuska JL, Holt A. Bioactive Leachates from Lab Plastics. G.I.T. Laboratory Journal 2009; 9-10: 2-4.
[6] Olivieri A, Degenhardt OS, McDonald GR, Narang D, Paulsen IM, Kozuska JL, Holt A. On the disruption of biochemical and biological assays by chemicals leaching from disposable laboratory plasticware.
Can J Physiol Pharmacol 2012; 90(6):697-703.
[7] Watson J, Greenough EB, Leet JE, Ford MJ, Drexler DM, Belcastro JV, Herbst JJ, Chatterjee M, Banks M. Extraction, identification, and functional characterization of a bioactive substance from automated compound-handling plastic tips. J Biomol Screen 2009; 14(5):566-72.
[8] Lewis LK, Robson M, Vecherkina Y, Ji C, Beall G. Interference with spectrophotometric analysis of nucleic acids and proteins by leaching of chemicals from plastic tubes. Biotechniques 2010; 48(4):297-302.
How to trick the Trojan horse?
To mitigate the potential impact of leachables, it is important to use high-quality consumables from reputable manufacturers that provide documentation on the materials used, their composition, and potential leachables. It is also advisable to conduct appropriate validation or characterization studies to assess the potential presence and impact of leachables on specific assays or experiments. Additionally, using suitable controls, blanks, or reference materials can help identify and quantify potential leachables in samples or reagents. Significant reduction of such sources of error and applicational risks might be achieved by using high quality plates, such as the Eppendorf PCR plates.
What you need to know
- Lab consumables are often overlooked as potential sources of error in assays such as PCR and NGS, with only assay-specific factors being properly controlled such as sample material, reagents, and equipment.
- Scientists need to optimize and validate each component of their workflow, including consumables, as they all work together, and their impact may vary depending on the specific setting.
- There is a growing recognition that consumables can negatively affect assay reproducibility.
- Leachables can affect the integrity, purity, or functionality of samples or reagents and interfere with a broad range of assays.
- It is crucial to use high-quality consumables, conduct validation studies, and employ suitable controls to mitigate leaching effects.
- By investing in high-quality plates like the Eppendorf PCR plates, scientists can significantly reduce the risk of leaching effects and increase the accuracy and efficiency of their experiments, ultimately leading to reliable and valid data.
Literature
[1] Lewis LK, Robson M, Vecherkina Y, Ji C, Beall G. Interference with spectrophotometric analysis of nucleic acids and proteins by leaching of chemicals from plastic tubes. Biotechniques 2010; 48(4):297-302. [2] Grzeskowiak R. Extractables and Leachables in Microcentrifuge Tubes – Extensive HPLC/GC/MS Analysis. Eppendorf AG Application Note. 2018: No. 417; www.eppendorf.com[3] McDonald GR, Hudson AL, Dunn SM, You H, Baker GB, Whittal RM, Martin JW, Jha A, Edmondson DE, Holt A. Bioactive contaminants leach from disposable laboratory plasticware. Science 2008; 322(5903):917
[4] Pfaffl M. Afif M. How to apply the MIQE Guidelines – a visual, interactive and practical qPCR guide! iBook (ISBN-9783000488061)
[5] McDonald GR, Kozuska JL, Holt A. Bioactive Leachates from Lab Plastics. G.I.T. Laboratory Journal 2009; 9-10: 2-4.
[6] Olivieri A, Degenhardt OS, McDonald GR, Narang D, Paulsen IM, Kozuska JL, Holt A. On the disruption of biochemical and biological assays by chemicals leaching from disposable laboratory plasticware.
Can J Physiol Pharmacol 2012; 90(6):697-703.
[7] Watson J, Greenough EB, Leet JE, Ford MJ, Drexler DM, Belcastro JV, Herbst JJ, Chatterjee M, Banks M. Extraction, identification, and functional characterization of a bioactive substance from automated compound-handling plastic tips. J Biomol Screen 2009; 14(5):566-72.
[8] Lewis LK, Robson M, Vecherkina Y, Ji C, Beall G. Interference with spectrophotometric analysis of nucleic acids and proteins by leaching of chemicals from plastic tubes. Biotechniques 2010; 48(4):297-302.
Read more
Read less
Related links
Read more
Read less



