MENU
AR | ARS
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
-
-
-
-
- Forum Labo 2025
- Advanced Therapies Week (ATW) 2025
- SLAS Europe 2025
- Bioprocessing Summit Europe 2025
- Medlab Middle East 2025
- SLAS International 2025
- Biologics World Nordics 2025
- ASIA LABEX: The Lab Show 2025
- BioProcess International Europe 2025
- ISEV 2025
- Future Labs Live 2025
- Cell 2025
- ASIA LABEX: The Lab Show 2025
AR | ARS
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Automated Pipetting
- Bottle-Top Dispensers
- Pipette Controllers
- Pipette Tips
- Automation Consumables
- Dispenser & Pipette Accessories
- Automation Accessories
- Dispenser & Pipette Services
You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.
Sorry, we couldn't find anything on our website containing your search term.

A Low Oxygen Atmosphere Supports the Xeno-Free Generation, Expansion, and Differentiation of Human Induced Pluripotent Stem Cells
RICK COHEN
We previously demonstrated that using low oxygen tension increased the efficiency of reprogramming human somatic cells to pluripotency [1].
In this study, we extend our findings and further increased the development of this culture paradigm. We are able to observe that post-electroporated fibroblasts cultivated at 4 % O2 grow on synthetic and biological surfaces. Further, we observed normal spreading of iPSCs and a high level of purity in early iPSC passages. The cells were successfully differentiated into motor neuron and cardiomyocyte lineages emphasizing the efficacy of low oxygen levels during cell cultivation.
In this study, we extend our findings and further increased the development of this culture paradigm. We are able to observe that post-electroporated fibroblasts cultivated at 4 % O2 grow on synthetic and biological surfaces. Further, we observed normal spreading of iPSCs and a high level of purity in early iPSC passages. The cells were successfully differentiated into motor neuron and cardiomyocyte lineages emphasizing the efficacy of low oxygen levels during cell cultivation.
Read more
Read less
Introduction
Reprogramming human somatic cells into the pluripotent state is a subject of thousands of publications since their discovery in 2007. Originally these studies were pioneered with a set of four genes found to be expressed in native human embryonic stem cells; Oct4, Sox2, KLF4, and c-Myc (Lin28 and Nanog), delivered using genetically modifying methods such as retrovirus, and cultured on non-defined matrices.
In the 13 years since these seminal studies many improvements were made such as (1) replacing genetically modifying methods with non-genome altering safer alternatives; (2) replacement of c-Myc oncogene with non-transforming family member, L-Myc; (3) inclusion of small molecules to boost efficiency of reprogramming; (4) optimizing culture conditions which includes the use of low O2 tension (4–5 %); and (5) use of clinically relevant defined media and matrices.
In this novel study, we combined many of the improvements to demonstrate the successful reprogramming of human foreskin fibroblasts with low O2 conditions in the CellXpert® C170i CO2 incubator. We observed that the tested growth substrates offered stable cell adhesion and spreading following electroporation of fibroblasts, which in turn led to a robust production of karyotypically normal iPSC colonies capable of robust expansion in defined media and ability to differentiate into neural and cardiac lineages.
Materials and methods
The reagents and procedures used in this Application Note* are similar to that described previously. When indicated, culture vessels were coated with 5 μg/mL of Vitronectin and used in comparison with a synthetic substrate. Once iPSC colonies appeared in Reprogramming Media, they were transitioned into Animal Free Low Protein hESC media for expansion. iPSC expansion and early steps in neuronal differentiation was carried out with 6-well plates, whereas cells analyzed for immunostaining or terminally differentiated to motor neurons or cardiomyocytes were spin seeded onto 24-well culture plates. Neurons were developed as described previously.
Results and discussion
Expansion and characterization of iPSCs under hypoxic conditions
Rudimentary iPSC colonies gave rise to more mature colonies at day 21–30 post modification and were passaged using gentle non-enzymatic methods. In order to purify the culture of Vitronectin derived cells, manual picking of colonies was used, whereas iPSCs grown on synthetic substrates outgrew any minor number of fibroblasts that were dislodged during the non-enzymatic passaging.
After 7 passages, the episomal plasmid reprogrammed line from the synthetic coating was analyzed for karyotype and found to be normal. Similar cultures were spin seeded onto 24-well dishes and analyzed for routine pluripotency or differentiation markers (Fig. 1). At passage 8, the cultures were found to lack any appreciable expression of SSEA1 and robustly expressed both SSEA4 and Oct4. Likewise, these cells coexpressed Lin28, Nanog, and Tra-1-60.
Reprogramming human somatic cells into the pluripotent state is a subject of thousands of publications since their discovery in 2007. Originally these studies were pioneered with a set of four genes found to be expressed in native human embryonic stem cells; Oct4, Sox2, KLF4, and c-Myc (Lin28 and Nanog), delivered using genetically modifying methods such as retrovirus, and cultured on non-defined matrices.
In the 13 years since these seminal studies many improvements were made such as (1) replacing genetically modifying methods with non-genome altering safer alternatives; (2) replacement of c-Myc oncogene with non-transforming family member, L-Myc; (3) inclusion of small molecules to boost efficiency of reprogramming; (4) optimizing culture conditions which includes the use of low O2 tension (4–5 %); and (5) use of clinically relevant defined media and matrices.
In this novel study, we combined many of the improvements to demonstrate the successful reprogramming of human foreskin fibroblasts with low O2 conditions in the CellXpert® C170i CO2 incubator. We observed that the tested growth substrates offered stable cell adhesion and spreading following electroporation of fibroblasts, which in turn led to a robust production of karyotypically normal iPSC colonies capable of robust expansion in defined media and ability to differentiate into neural and cardiac lineages.
Materials and methods
The reagents and procedures used in this Application Note* are similar to that described previously. When indicated, culture vessels were coated with 5 μg/mL of Vitronectin and used in comparison with a synthetic substrate. Once iPSC colonies appeared in Reprogramming Media, they were transitioned into Animal Free Low Protein hESC media for expansion. iPSC expansion and early steps in neuronal differentiation was carried out with 6-well plates, whereas cells analyzed for immunostaining or terminally differentiated to motor neurons or cardiomyocytes were spin seeded onto 24-well culture plates. Neurons were developed as described previously.
Results and discussion
Expansion and characterization of iPSCs under hypoxic conditions
Rudimentary iPSC colonies gave rise to more mature colonies at day 21–30 post modification and were passaged using gentle non-enzymatic methods. In order to purify the culture of Vitronectin derived cells, manual picking of colonies was used, whereas iPSCs grown on synthetic substrates outgrew any minor number of fibroblasts that were dislodged during the non-enzymatic passaging.
After 7 passages, the episomal plasmid reprogrammed line from the synthetic coating was analyzed for karyotype and found to be normal. Similar cultures were spin seeded onto 24-well dishes and analyzed for routine pluripotency or differentiation markers (Fig. 1). At passage 8, the cultures were found to lack any appreciable expression of SSEA1 and robustly expressed both SSEA4 and Oct4. Likewise, these cells coexpressed Lin28, Nanog, and Tra-1-60.
Read more
Read less
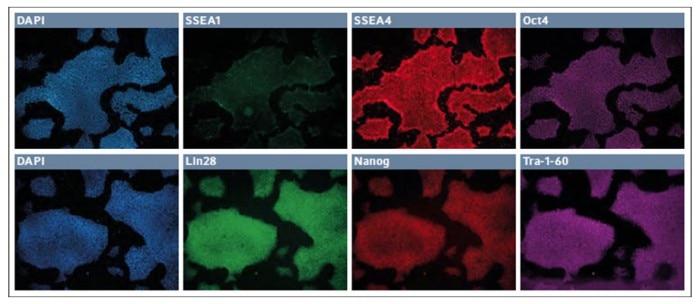
Fig. 1: Immunofluorescence staining of markers for pluripotency and differentiation in low passage iPSCs
Differentiation of iPSCs to neuronal and cardiomyocyte lineages
The iPSCs were first differentiated into neuroepithelial cells and then secondly into neural stem cells. After 4 passages in neural stem cell media, the cells appeared to differentiate into a uniform mat, with repeating patterns of rosettes, characteristic of neural stem cell stage. Many of these cells were OTX2+, with a greater number expressing both Pax6 and Nestin.
Since this was the second step out of four to derived motor neurons, we continued to differentiate the stem cells into presumptive motor neuron precursors. The small molecules and growth factors were altered to continue the differentiation protocol (Fig. 2, upper panel), and some of the cells continued to express OTX2 and Nestin, while many began to express Olig2, a marker of motor neuron lineage.
After one week, the cells were finally exposed to a set of cytokines and small molecules to induce the final differentiation into presumptive motor neurons (Fig. 2, lower panel).
The iPSCs were first differentiated into neuroepithelial cells and then secondly into neural stem cells. After 4 passages in neural stem cell media, the cells appeared to differentiate into a uniform mat, with repeating patterns of rosettes, characteristic of neural stem cell stage. Many of these cells were OTX2+, with a greater number expressing both Pax6 and Nestin.
Since this was the second step out of four to derived motor neurons, we continued to differentiate the stem cells into presumptive motor neuron precursors. The small molecules and growth factors were altered to continue the differentiation protocol (Fig. 2, upper panel), and some of the cells continued to express OTX2 and Nestin, while many began to express Olig2, a marker of motor neuron lineage.
After one week, the cells were finally exposed to a set of cytokines and small molecules to induce the final differentiation into presumptive motor neurons (Fig. 2, lower panel).
Read more
Read less
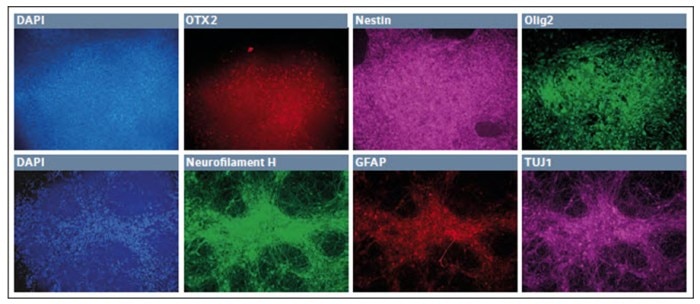
Fig. 2: Differentiation of iPSCs into presumptive motor neuron precursor cells in low O2 environment
After 7 days in the final media cocktail, the cells organized a fine network of process which were robustly Neurofilament H+, with some remaining TUJ1+ appearance and few GFAP+ glial cells. Together these results showed that hypoxic incubation can be used to derive cells of neuroectodermal lineage. In addition, we were able to first differentiate the cells into definitive endoderm and then finally develop them into cardiomyocytes (Fig. 3).
Read more
Read less
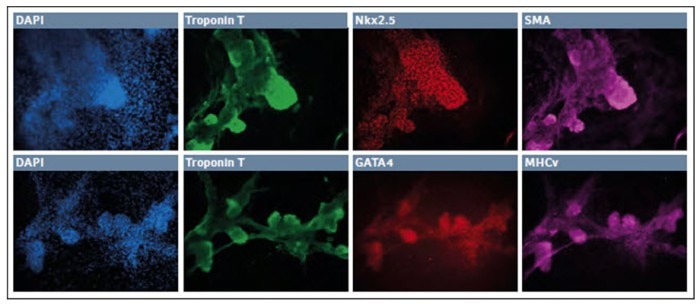
Fig. 3: Differentiation of iPSCs into cardiomyocytes in low O2 environment
After 10–11 days in culture the surviving cells organized into small patches. These “nodes” were highly three dimensional and expressed Troponin T, Nkx2.5, and SMA. Further, we observed robust expression of Troponin T along with GATA4 and MHCv. Together this indicates the successful development of the cardiomyocyte lineage in a hypoxic environment.
Conclusion
We successfully demonstrated the reprogramming of human foreskin fibroblasts in low oxygen tension in the CellXpert C170i CO2 incubator. The iPSC line was characterized as karyotypically normal and found to express expected markers of pluripotency. Further, the low O2 environment supported the reliable differentiation of iPSCs into various stages of ectoderm, neuronal stem cells, presumptive motor neuron precursors, and finally motor neurons. As well, the hypoxic atmosphere inside the incubator supported the differentiation of the same iPSC line into cardiomyocytes.
*Download the full Application Note 443
Literature
[1] Low Oxygen Levels Enhance the Efficiency of Reprogramming Human Somatic Cells to Pluripotency. Eppendorf Application Note 338 .
Note: Eppendorf SE reserves the right to modify its products and services at any time. This Application Note is subject to change without notice. Although prepared to ensure accuracy, Eppendorf SE assumes no liability for errors, or for any damages resulting from the application or use of this information. Viewing Application Notes alone cannot as such provide for or replace reading and respecting the current version of the operating manual.
Conclusion
We successfully demonstrated the reprogramming of human foreskin fibroblasts in low oxygen tension in the CellXpert C170i CO2 incubator. The iPSC line was characterized as karyotypically normal and found to express expected markers of pluripotency. Further, the low O2 environment supported the reliable differentiation of iPSCs into various stages of ectoderm, neuronal stem cells, presumptive motor neuron precursors, and finally motor neurons. As well, the hypoxic atmosphere inside the incubator supported the differentiation of the same iPSC line into cardiomyocytes.
*Download the full Application Note 443
Literature
[1] Low Oxygen Levels Enhance the Efficiency of Reprogramming Human Somatic Cells to Pluripotency. Eppendorf Application Note 338 .
Note: Eppendorf SE reserves the right to modify its products and services at any time. This Application Note is subject to change without notice. Although prepared to ensure accuracy, Eppendorf SE assumes no liability for errors, or for any damages resulting from the application or use of this information. Viewing Application Notes alone cannot as such provide for or replace reading and respecting the current version of the operating manual.
Read more
Read less
