-
- Tischzentrifugen
- Standzentrifugen
- Gekühlte Zentrifugen
- Mikrozentrifugen
- Mehrzweckzentrifugen
- Hochgeschwindigkeitszentrifugen
- Ultrazentrifugen
- Concentrator
- IVD Produkte
- High-Speed and Ultracentrifuge Consumables
- Zentrifugenröhrchen
- Zentrifugenplatten
- Gerätemanagement
- Proben- und Informationsmanagement
-
- Manuelles Pipettieren & Dispensieren
- Mechanische Pipetten
- Elektronische Pipetten
- Mehrkanalpipetten
- Direktverdrängerpipetten & Dispenser
- Automatisches Pipettieren
- Flaschenaufsatzdispenser
- Pipettierhilfen
- Pipettenspitzen
- Verbrauchsartikel für die Automation
- Zubehör für Dispenser & Pipetten
- Zubehör für die Automation
- Services für Dispenser & Pipetten

Biosafety at the Highest Level
Lab Academy
- Pharma
- Biotechnology
- Health & Medicine
- Lab Life
- Lab Routine
- Pipetting & Dispensing
- Efficiency
- countonit
- Reproducibility
- Ergonomics
- Automation
- Essay
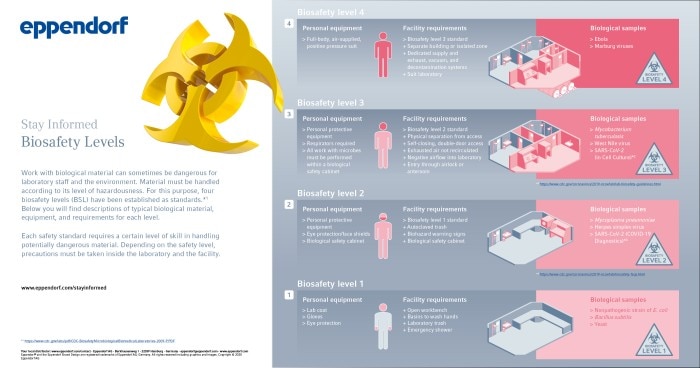
The Bernhard Nocht Institute for Tropical Medicine (BNITM) is Germany’s largest institution for research, treatment, and training in the field of tropical diseases and new emerging infections. The researchers at this world-famous institution perform clinical studies, epidemiology, and disease control, as well as investigating the biology of pathogens, their reservoirs, and vectors. For the handling of highly pathogenic viruses and infected insects, the Institute has laboratories of all biosafety levels (BSL), up to the highest level 4 (BSL-4). A laboratory of this kind is designed to diagnose and investigate extremely hazardous pathogens like those causing Ebola, Crimean-Congo haemorrhagic fever, Marburg disease, or Lassa fever without endangering the staff or the population at large. Across the globe, there are several dozen BSL-4 laboratories. Learn from the experience of top specialists how to perfect sample safety in your laboratory.
Mehr erfahren
Weniger lesen
Reliable, accurate operating procedures are extremely important, especially when working with cell cultures - and this applies even more so under the tough conditions of a maximum safety laboratory (Webinar Protect Your Cells with Proper Pipetting) . A challenge faced by any cell culturist is the contamination of cell cultures. But for researchers who work with the most dangerous and scarce specimens, it is most crucial to minimize this problem with care, mindfulness, and good aseptic techniques, supported by the right choice of equipment, which can make a huge difference in your daily workflow (Webinar Preventing Contamination in Cell Culture Labs) . In order to prevent cross-contamination and to save extremely valuable lab-time, the researchers arrange the required pipettes and consumables in method-specific sets.
As vision is impaired by two to three layers of protection (individual glasses, protective visor, and the glass pane of the clean bench) the tips for multichannel pipettes should be as high-contrast as possible, explains Dr. Toni Rieger, BioRisk Manager at the BNI: “This is a very important issue for us. To make sure not to miss the correct well, we work perhaps even more carefully and more slowly with the agents in the BSL-4 laboratory than we would perform similar tasks in level 1 or 2 laboratories. And, especially with multichannel pipettes, we have to make sure that all tips are fitted correctly and absorb equal volume. We have to keep a good eye on them and take regular breaks, so that our eyes and concentration don’t get weary.”
And it is not only the eyes that tire quickly under these conditions: working in a protective PVC suit with three pairs of tight-fitting Nitrile gloves on top of each other is much more physically strenuous than in a normal laboratory. This is especially hard on the hands when pipetting over an extended period of time. So, if available, pipetting robots and automation can relieve many issues – from working conditions to sample safety.
Mehr erfahren
Weniger lesen
One illustration of the importance of a well-trained staff, careful preparation, and the right choice of equipment is the handling of hazardous aerosols; another one is the cleaning of equipment that has come into contact with hazardous biomaterials.
Aerosols (Stay Informed Aerosols in the Lab) are tiny liquid drops evaporating naturally from each liquid containing contamination such as viruses, bacteria, or molecules like proteins, DNA, or RNA. The liquid drops rise into the pipette cones, which can lead to internal contaminations of the pipette and cross-contamination of multiple samples, ruining entire applications. In the BSL-4 laboratory, the use of filter tips is mandatory. Pipette tips containing a filter can prevent aerosols from entering the pipette cone, and special blocking filters made of swelling material block the tip when the filter comes in contact with liquids.
Aerosols are an issue with centrifugation as well, says Dr. Rieger: “The centrifuges' rotors have aerosol-tight lids, which must only be opened under the clean bench.”
Mehr erfahren
Weniger lesen
“Once a year, the entire laboratory and all tools and devices are cleaned, wipe disinfected, and fumigated with hydrogen peroxide,” explains BioRisk manager Rieger. In this context, the maintenance and calibration of the pipettes is also carried out by an external maintenance service. The surfaces of the pipettes are wipe disinfected after each use. “We always work with filter tips and our staff are very experienced, so it is very rare for a pipette to be contaminated in the BSL-4 laboratory. However, as we do not have running water in this laboratory, we cannot rinse the pipettes,” says Rieger. Nevertheless, once a pipette is contaminated, it needs to be cleaned. Since any exchange with the environment has to be limited to the absolute minimum in laboratories of the highest safety level, contaminated pipettes are replaced and stored for the annual review. If this is not feasible, they would have to be discharged via the autoclave or fumigation with hydrogen peroxide, then thoroughly cleaned and rinsed, before being reintroduced to the lab.
In other laboratories, it is advantageous if pipettes can be thoroughly cleaned by the users. To meet this specification, a pipette must meet a number of requirements: The lower part of pipettes should be easily removable and cleanable by rinsing on the inside and with thorough cleaning of the whole pipette. This is especially important when using electronic pipettes, as there is no standard yet. The pipette has to be resistant to common cleaning agents and DNA/RNA cleaning solutions.
If sterility is an issue, the pipette must be autoclavable without dismounting. When using Eppendorf electronic pipettes, the lower part is removable and autoclavable to inactivate microorganisms that entered the pipette cone. If a pipette gets damaged in a BSL-4 laboratory, there are three possible solutions, depending on the damage: Repair by the users, repair by the maintenance service in the annual review, or destruction.
Mehr erfahren
Weniger lesen
Just as important as the quality of the pipette itself is the quality of the tips, as they are perfectly matched within a system. (Application Note No. 354: The Tip of the Iceberg) . Conical tubes (Application Notes No. 427: Optimization of Bacterial Culture and Plasmid Purification with Eppendorf Conical Tubes 25 m) with tight lid closure and safe handling, as well as centrifugation stability (Application Notes No. 343: Performance comparison of Eppendorf Conical Tubes) , are of extreme importance for optimal sample accessibility combined with minimal risk of cross contamination or spillage. Scarce samples can be protected from evaporation with safe-lock tubes (Application Note No. 164: Eppendorf Safe-Lock Tubes) . As UV-absorbing bioactive contaminants leaching out of laboratory consumables (Application Note No. 396: Is That Really DNA in Your Tube?) may significantly affect experiments and pose a likely source of error in many assay systems, the laboratory operator should carefully choose the proper test tubes to ascertain that the scarce specimens are not wasted due to incorrect readings.
Specimen materials are often extremely scarce in critically ill patients with rare or novel infectious pathogens. Thus, they must not be wasted by flawed experiments under any circumstances (Quality of Results).
Mehr erfahren
Weniger lesen
“In such an exceptional working environment as a BSL-4 laboratory, attention to detail is one of the key success factors. We depend on each other and on everyone working carefully, collegially and responsibly,” says Dr. Rieger. "We all have to work at the same site with the same equipment, which has to be treated with care and left behind in an orderly fashion. This applies to the shared devices as well as to each individual pipette. We meet regularly to improve our collegial working methods and processes. This is an approach that can benefit every laboratory team.”
Find out more click here.
Mehr erfahren
Weniger lesen
More information
Mehr erfahren
Weniger lesen

