MENÜ
DE | EUR
DE | EUR
-
- Tischzentrifugen
- Standzentrifugen
- Gekühlte Zentrifugen
- Mikrozentrifugen
- Mehrzweckzentrifugen
- Hochgeschwindigkeitszentrifugen
- Ultrazentrifugen
- Concentrator
- IVD Produkte
- High-Speed and Ultracentrifuge Consumables
- Zentrifugenröhrchen
- Zentrifugenplatten
- Gerätemanagement
- Proben- und Informationsmanagement
-
- Manuelles Pipettieren & Dispensieren
- Mechanische Pipetten
- Elektronische Pipetten
- Mehrkanalpipetten
- Direktverdrängerpipetten & Dispenser
- Automatisches Pipettieren
- Flaschenaufsatzdispenser
- Pipettierhilfen
- Pipettenspitzen
- Verbrauchsartikel für die Automation
- Zubehör für Dispenser & Pipetten
- Zubehör für die Automation
- Services für Dispenser & Pipetten
Es konnten keine Ergebnisse gefunden werden.
Such-Empfehlungen

PCR Workflows: Quality Enhancement Through Standardization
Lab Academy
- Molecular Biology
- Amplification & PCR
- FAQ
- Essay
The standardization of processes includes their optimization and subsequent establishment and harmonization, allowing long-term optimal performance – independent of the user. Standardization ensures high-quality results, as well as their reproducibility and comparability.
The goal of (classic) PCR is the generation of a reliable and reproducible result. For certain applications, the yield of the PCR product is also relevant. For these reactions, care must be taken to ensure that samples are not compromised and that the PCR workflow remains stable. Specifically, this translates to minimizing the introduction of contaminations that could lead to false positive or false negative results or even inhibit the PCR reaction. Furthermore, the reaction conditions should be as identical as possible for each individual sample within a run and also be transferable to subsequent reactions (of the same method). This refers to the composition of the reactions as well as to the type of temperature control in the cycler. User errors, of course, are to be avoided as much as possible.
Below, we will demonstrate the challenges which are encountered during preparation and throughout the run of a PCR – and the approaches to solutions which exist with respect to instruments and consumables used for the standardization of the PCR workflows.
The dispensing of reaction components into PCR-vessels, or plates, respectively, comprises multiple challenges that must be overcome:
The exact and precise dosing of the individual components is indispensable when aiming for the most identical reaction conditions possible. In addition to a good pipetting technique, selecting the right tool is critical. PCR master-mixes frequently contain substances which increase viscosity or generate foam. During the pipetting process, these lead to substantial wetting of the pipette tips, thus reducing pipetting accuracy. The use of direct dispensing systems (figure 1) or alternative pipette tips that are less prone to wetting (figure 2) can improve the accuracy and precision of the pipetting process. (Pipetting PCR mixtures)
If a large number of PCRs are performed, it is also worth considering automation of the setup. (Automated PCR Set-up)
Below, we will demonstrate the challenges which are encountered during preparation and throughout the run of a PCR – and the approaches to solutions which exist with respect to instruments and consumables used for the standardization of the PCR workflows.
Reaction preparation
The dispensing of reaction components into PCR-vessels, or plates, respectively, comprises multiple challenges that must be overcome:
Reaction conditions
The exact and precise dosing of the individual components is indispensable when aiming for the most identical reaction conditions possible. In addition to a good pipetting technique, selecting the right tool is critical. PCR master-mixes frequently contain substances which increase viscosity or generate foam. During the pipetting process, these lead to substantial wetting of the pipette tips, thus reducing pipetting accuracy. The use of direct dispensing systems (figure 1) or alternative pipette tips that are less prone to wetting (figure 2) can improve the accuracy and precision of the pipetting process. (Pipetting PCR mixtures) If a large number of PCRs are performed, it is also worth considering automation of the setup. (Automated PCR Set-up)
Mehr erfahren
Weniger lesen
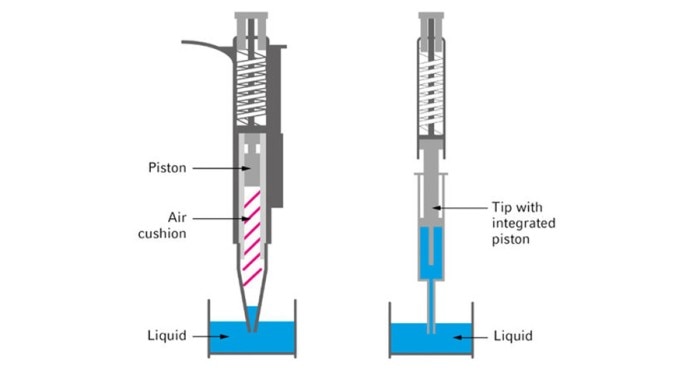
Figure 1: Aircushion (left) vs. direct displacement system (right)
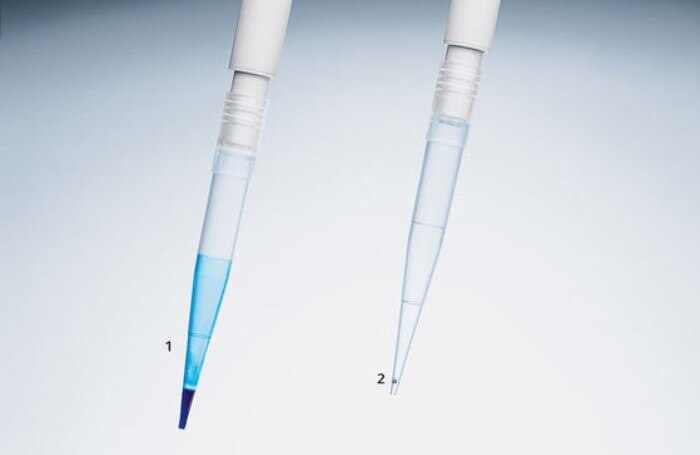
Figure 2: Wetting of pipette tips after pipetting of a solution containing detergents (like PCR master mix); Pipette tip 2 shows a LoRetention tip that reduces attachment of detergents to the tip wall.
Contaminations
During the dispensing process, aerosols are generated which, if allowed to reach the inside of the pipette, can potentially contaminate another sample during the next pipetting step. This can be prevented by using filter tips (figure 3) or direct displacement systems.Consumables such as tips, vessels and plates that are used in the PCR workflow must not contain substances which compromise the sample or falsify the result. These include DNA, DNases, RNases and PCR inhibitors (figure 4), as well as components that may potentially leach from the material during the reaction – substances known as leachables [1].
Mehr erfahren
Weniger lesen
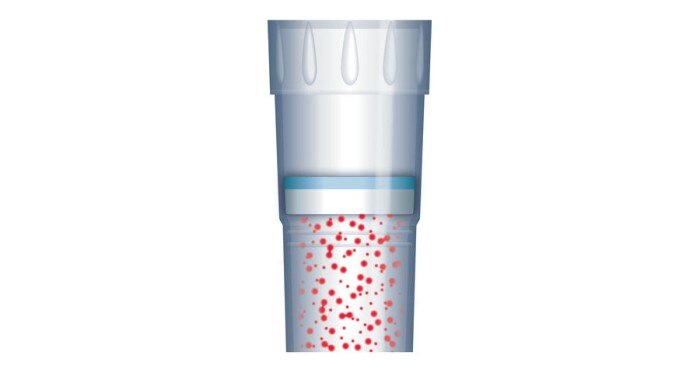
Figure 3: ep Dualfilter T.I.P.S. ® provide protection of pipettes and samples from contamination by aerosols, drops and splashes

Figure 4: Eppendorf purity grade PCR clean: certified free of DNase, RNase, human DNA, PCR inhibitors
User error
The more samples are processed, the higher the error risk. It can easily happen that a sample is pipetted into the wrong vessel or the wrong well. This risk can be considerably reduced by easily discernible marking of the wells (figure 5). Through the automation of dispensing steps, the “human factor”, i.e., errors and user-related variations, are minimized, thus increasing reproducibility, especially in the case of small reaction volumes [2, 3]. This requires plates of sufficient dimensional stability to be employed in a workstation. Attached barcodes provide additional machine-readability, which simplifies sample tracking throughout the entire process (figure 6). Mehr erfahren
Weniger lesen

Figure 5: Eppendorf twin.tec® PCR plates with OptiTrack® labeling for easy identification of wells.

Figure 6: Eppendorf twin.tec® PCR plates with barcode for sample tracking.
Programming of the thermocycler
Programming an instrument can prove to be time-consuming as well as error-prone. Different PCR thermal cycler features work together to simplify this process step and, most importantly, to make it safe:Easy operation and good user guidance are the basis of efficient programming. Building on this foundation, password-protected user administration will prevent one’s own programs from being altered by other users. If multiple cyclers (of the same type) are in use, it is beneficial if a program can be transferred directly from one instrument to another via USB or connectivity. Computer software enables central and secure administration of programs, user rights and documents on a computer.
PCR run
During the run, DNA is amplified in the reaction vessel, where each sample should be subjected to identical, consistent reaction conditions. The following aspects are relevant for the process:
Temperature control
Excellent accuracy in temperature control and homogeneity of the cycler block are the basis for even temperature conditioning of all samples. The high quality of the heating and cooling elements (peltier elements), as well as the way in which these are connected to the block, are the deciding factors which will determine the risk of temperature discrepancies known as the “edge effect” (figure 7) ( Peltiers in PCR ) Mehr erfahren
Weniger lesen
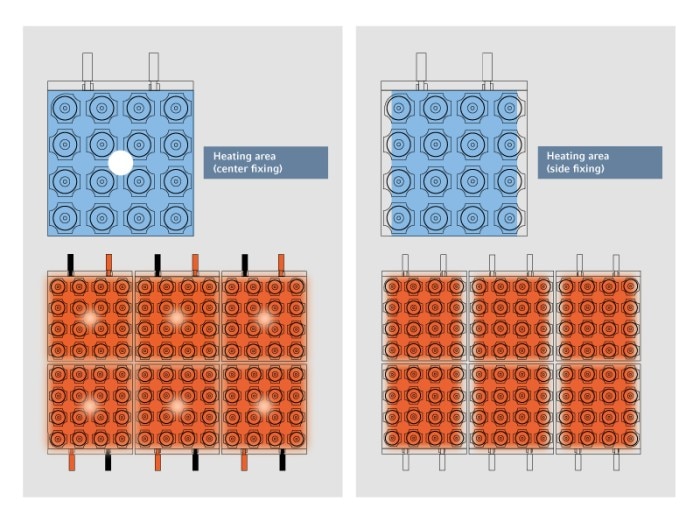
Figure 7: Heating areas (shaded orange) of the thermal block when fixing peltiers at the center vs. heating areas of the thermal block when fixing peltiers at the sides
Evaporation
The concentrations of the individual reaction components should not change over the course of the reaction due to evaporation. Otherwise, it is possible that very little PCR product may be generated, or none at all. It is therefore crucial to minimize evaporation by ensuring a secure seal. In this case, the heated lid of the thermocycler and the seal of the vessel work hand in hand. Different sealing options are available for PCR plates (link: Sealing article), whereby the best seal is achieved through heat sealing. Other closures may also be suitable, as long as the contact pressure of the cycler lid can be adjusted to the selected seal.Process standardization is in place in order to safeguard accurate and reproducible results in the long term. This includes regular maintenance of the equipment to ensure that it is always in good working condition. All consumables should be of consistently high quality across all lots produced, and their reliable availability must be guaranteed.
References:
[1] White Paper 26
[2] Application Note 368
[3] Application Note 388
Mehr erfahren
Weniger lesen
Mehr erfahren
Weniger lesen
