-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
- Service pour pipette
-
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
- Service pour pipette
-
-
- Centrifugeuses de paillasse
- Centrifugeuses au sol
- Centrifugeuses réfrigérées
- Microcentrifugeuses
- Centrifugeuses multi-fonctions
- Centrifugeuses haute vitesse
- Ultracentrifugeuses
- Concentrateur
- Produits IVD
- High-Speed and Ultracentrifuge Consumables
- Tubes de centrifugeuse
- Plaques de centrifugeuse
- Gestion des appareils
- Gestion des échantillons et des informations
-
- Pipetage manuel & distribution
- Pipettes mécaniques
- Pipettes électroniques
- Pipettes multicanaux
- Distributeurs et pipettes à déplacement positif
- Automates de pipetage
- Distributeurs sur flacon
- Auxiliaires de pipetage
- Pointes de pipette
- Consommables d’automatisation
- Accessoires pour pipettes et distributeurs
- Accessoires d’automatisation
- Services pour pipettes et distributeurs
Bioprocess Operation Modes: Batch, Fed-batch, and Continuous Culture
Overview
Feed automation
Perfusion
Lire moins
What are the differences between batch, fed-batch, and continuous culture?
Lire moins
Batch culture
In a batch culture, substrate is administered at the start of the bioprocess, with no further additions. The feed is gradually consumed by the culture and there is an accumulation of by-products over time. When the cells enter the stationary growth phase, the culture is harvested.
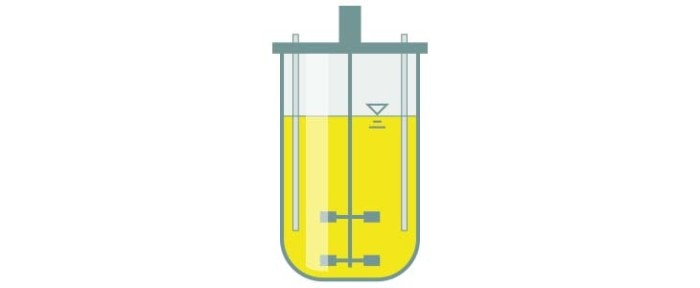
- Simple operation and low risk of contamination
- Suitable for the early stages of experimental design
- Relatively short duration
- Relatively low cell densities and yields
- Toxic by-products accumulate over time
Lire moins
Fed-batch culture
In a fed-batch culture, batch bioprocessing is conducted for a certain period of time. Then, to counter any decline in cell growth or cell densities, additional substrate is added in increments throughout the rest of the bioprocess.
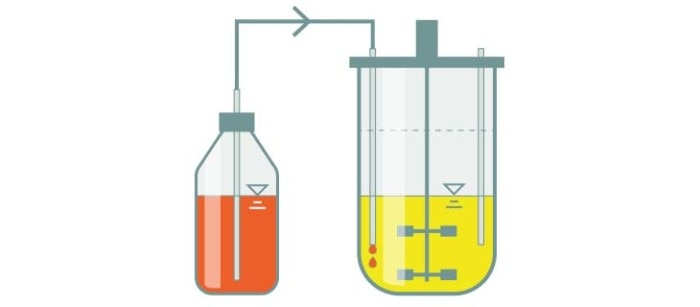
- The most widely used bioprocess mode
- Compared to batch fermentation:
- A higher cell density can be achieved
- Extended duration of the cell culture
- Less substrate overfeeding is required at the start of the culture, to decrease the accumulation of toxic by-products and improve cell growth
- Incremental feeding following the batch fermentation period increases the risk of contamination
- Harvesting is only carried out at the end of the process and so there is a lack of continuous by-product removal
Lire moins
Continuous culture
In a continuous culture, substrate is continuously added to the culture, with the continuous harvesting of cells and culture medium. Like this, nutrients are replenished and by-products removed. The culture volume remains constant when feeding and harvesting are carried out at the same rate. This allows for a steady state to be achieved and maintains a constant environment and rate of cell growth.
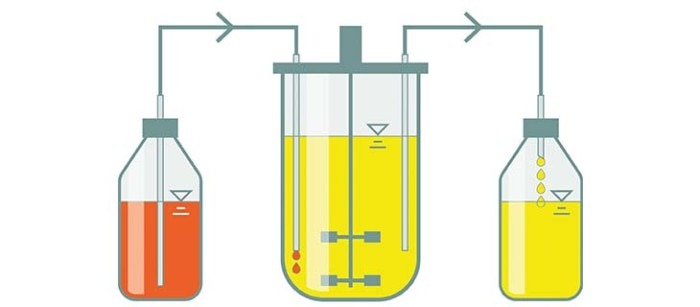
- A constant growth environment can be maintained, which can positively influence the product quality
- Steady state cultures can be maintained for up to several months to reduce the culture downtime
- Process operation is more challenging than in batch or fed-batch processes
- Increased risk of contamination due to the extended duration and continuous feeding and harvesting
- Limited traceability, as the culture suspensions aren’t harvested in batches
Lire moins
Perfusion culture
Perfusion culture is a type of continuous culture where cells are retained or recycled back into the bioreactor. Similar to other types of continuous culture, nutrients are replenished, and toxic by-products are removed to keep the culture in a steady state.
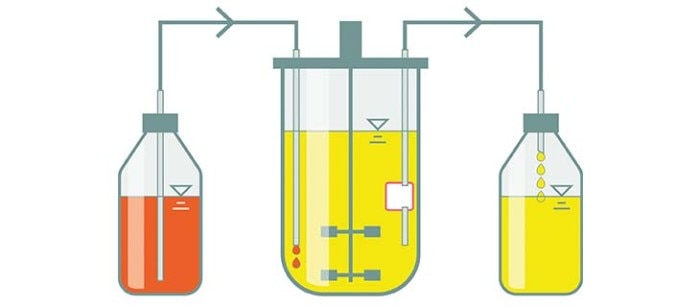
- Steady state cultures can be maintained for up to several months to reduce the culture downtime
- High cell densities - and subsequently high yields and volumetric productivity can be achieved
- Due to higher volumetric productivity the working volume and therefore equipment footprint can be reduced, compared to batch or fed-batch processes
- Process operation is more challenging than in batch or fed-batch processes
- Increased risk of contamination due to the extended duration and continuous feeding and harvesting
- Limited traceability, as the culture suspensions aren’t harvested in batches
Lire moins
Which bioprocess mode is best suited for your application?
Lire moins
How can feed automation improve bioprocesses? And what is required to establish automated feeding?
Sensor integration
Lire moins
Strategies for feed automation
Lire moins
Perfusion cell culture: What are cell retention techniques for perfusion culture and which devices can be used for these?
Lire moins
Filtration using spin filters
Filtration using spin filters
Lire moins
Get more information on how a spin filter functions.

Lire moins
Tangential flow filtration (ATF and TFF)
Lire moins
Tangential flow filtration (TFF)
Lire moins
Alternating tangential flow (ATF) filtration
Lire moins
Perfusion process development at small scale
Lire moins
- Using the DASbox® Mini Bioreactor System , ATF perfusion cultivation of CHO cells and HEK293 cells in a working volume as low as 230 mL was established.
Lire moins
- Using a DASGIP® Parallel Bioreactor System , CHO cells were cultivated in perfusion in a working volume of 1 L.
Lire moins
Packed-bed bioreactors
Packed-bed bioreactors are filled with a solid growth support on or in which the cells are immobilized. One such support are Fibra-Cel® discs . Fibra-Cel is a porous meshwork of polyester and polypropylene that provides a growth surface for adherent cells and entraps suspended cells. Immobilization of cells in the matrix facilitates the easy harvest of cell-free medium from the bioreactor. Furthermore, Fibra-Cel® offers a high surface to volume ratio and protects cells from damaging shear forces.

Lire moins
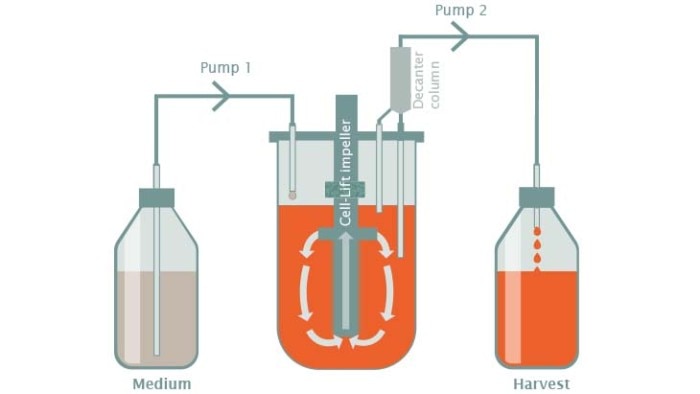
Cell-Lift impeller with decanter column
Cell-Lift impellers with decanter columns can be used for perfusion cultivation of cells grown on microcarriers. Cell-Lift impeller rotation creates a negative pressure in the hollow impeller tube causing medium to circulate uniformly in a closed loop. Cells and microcarriers are separated from the medium in the decanter column. The medium is harvested and the cells and microcarriers are transferred back to the bioreactor. Advantages of this system include reduced shear force, high mass transfer of nutrients, and a high oxygen transfer rate.
Lire moins
Lire moins


