MENU
FR | EUR
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
- Service pour pipette
-
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
- Service pour pipette
-
FR | EUR
-
- Centrifugeuses de paillasse
- Centrifugeuses au sol
- Centrifugeuses réfrigérées
- Microcentrifugeuses
- Centrifugeuses multi-fonctions
- Centrifugeuses haute vitesse
- Ultracentrifugeuses
- Concentrateur
- Produits IVD
- High-Speed and Ultracentrifuge Consumables
- Tubes de centrifugeuse
- Plaques de centrifugeuse
- Gestion des appareils
- Gestion des échantillons et des informations
-
- Pipetage manuel & distribution
- Pipettes mécaniques
- Pipettes électroniques
- Pipettes multicanaux
- Distributeurs et pipettes à déplacement positif
- Automates de pipetage
- Distributeurs sur flacon
- Auxiliaires de pipetage
- Pointes de pipette
- Consommables d’automatisation
- Accessoires pour pipettes et distributeurs
- Accessoires d’automatisation
- Services pour pipettes et distributeurs
Aucun résultat trouvé
Chercher des suggestions
Stem Cells - Scientific Background
Lab Academy
- Bioprocédés
- Biologie cellulaire
- Culture cellulaire
- Cellules souches
- Incubateurs à CO2
- Bioprocédés
- Consommables pour culture cellulaire
- Test
What are stem cells?
Defining what a stem cell is has been challenging due to the variation in properties of all the cells that could be described as stem cells.
When most people think of stem cells, they think of a single cell type that can differentiate into all cell types of an organism. However, this definition would be a better fit for embryonic stem cells, as there are stem cells that can only differentiate into a limited number of cell types with a similar lineage, or developmental history from embryo to mature cell.
Lire moins
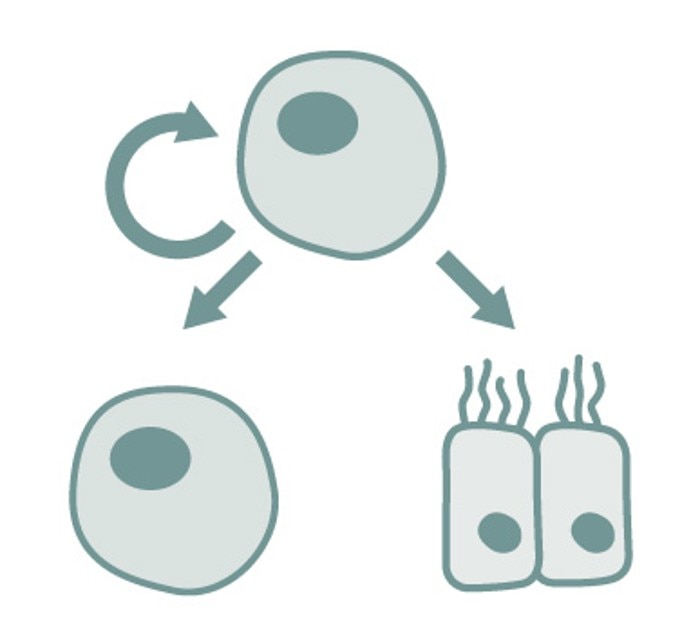
A better way to describe stem cells is by looking at the characteristics that all stem cells have in common. These characteristics are that they are (1) not fully differentiated yet, (2) able to differentiate into multiple specialized mature cell types, and (3) capable of multiplying without losing their stem cell characteristics.
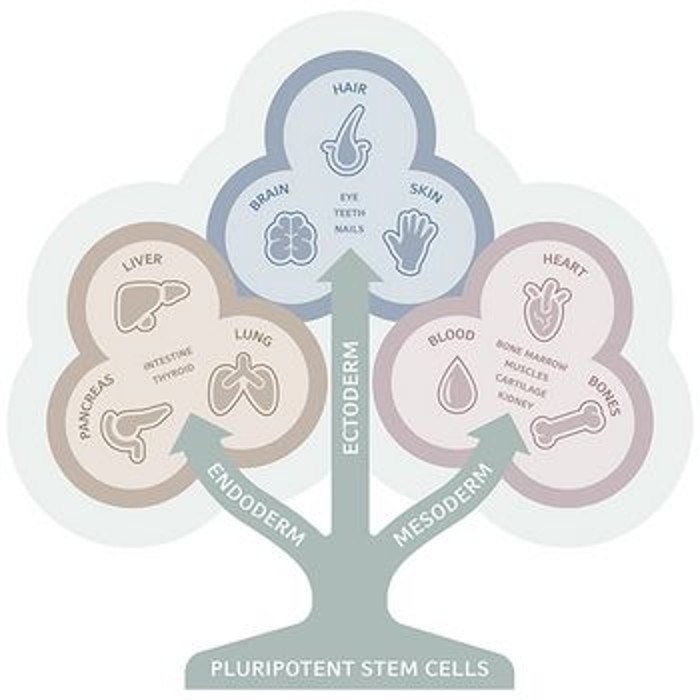
A common way of classifying stem cells is by their 'potency', which indicates the number of cell types into which the stem cell can differentiate. Pluripotency, for example, refers to cells that can differentiate into cells of all three germ layers of an organism: ectoderm, mesoderm and endoderm. Multipotent cells, on the other hand, can only differentiate into a limited number of (often closely related) cell types.
Lire moins
In research, the three most commonly used stem cells are embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs).
Types of stem cells
Embryonic stem cells
ESCs are present in the cores of early-stage embryos – typically in the first week after fertilization before implantation of the embryo has taken place. In more mature embryos, ECSs differentiate, first into three germ layers and then into more specialized cells. ESCs can be cultured in vitro where, under the right conditions, they have the ability to proliferate indefinitely without losing their pluripotency2 . These conditions include specialized culture media, incubators with highly reliable temperature and gas control (for example hypoxic culture), and growth .
The unlimited growth potential of ESCs, combined with their potential for differentiating into any cell type in the body, makes them an attractive option for use in research and in the clinic. Functional mature cells and tissues created from ESCs can replace or support damaged tissue – as well as provide a deeper insight into the development of embryos, tissues and organs.
However, the use of human ESCs comes with ethical considerations that go beyond the laboratory. The use of ESCs is ethically and politically controversial, because the derivation of ESCs involves the destruction of embryos. Therefore, several countries have regulated or banned the creation and use of human ESCs.
Nevertheless, in recent years over a thousand scientific papers about human ESCs have been published annually3 , and more than a dozen clinical trials using ESC-derived cells are currently underway with encouraging early results4 . The last decade has also witnessed the rise of iPSCs. These cells are a successful alternative to ESCs, which are created by 'reprogramming' of mature cells and therefore avoid many of the ethical issues surrounding the use of live embryos.
Induced pluripotent stem cells
iPSCs are a type of pluripotent cell, created by introducing pluripotency-related genes into the genome of mature, fully differentiated fibroblasts. When cells start expressing these genes, they undergo a transformation into ESC-like cells capable of differentiating into the same range of lineages.
The first were developed in 2006 by Japanese researchers Shinya Yamanaka and Kazutoshi Takahashi, who used retroviral transduction to introduce four genes (Oct3/4, Sox2, Klf4 and c-Myc) into mouse fibroblasts5 . The same group later showed significanthypoxic improvements in reprogramming efficiency when using conditions6 . Further modifications to the method by other researchers led to reprogramming of neural cells to iPSCs using only a single gene (Oct4)7 .
Although viral transduction is a relatively efficient method for generating iPSCs, other groups have studied alternative reprogramming strategies that could improve reliability by reducing genetic abnormalities and the risk of tumor formation. These strategies have focused on avoiding the use of viruses, avoiding the need for integrating DNA into the cells’ genome, or both. Examples of viral and non-viral methods include episomal vectors, adenoviral vectors, Sendai viral vectors, plasmids, synthetic mRNA, miRNA, protein transduction and small molecules8,9 .
Avoiding the ethical issues surrounding ESC isolation has been a key factor in the success of iPSCs. In addition to this, iPSCs also have potential for use in autologous, patient-specific treatments. Because iPSCs originate from adult cells, it is possible to generate iPSCs from a patient’s own cells, opening up possibilities for treatment not possible with non-autologous cells.
However, both ESCs and iPSCs carry important safety concerns in clinical trials. Because both cell types are pluripotent, they have the ability to form tumors when accidentally implanted in an undifferentiated state. This risk means that treatments using cells derived from ESCs or iPSCs need strict quality control to eliminate the risk of tumor formation.
In the years after their discovery, iPSCs have become one of the standard tools available to stem cell researchers, providing a valuable alternative to ESCs for in vitro studies and in therapeutic use10 .
Mesenchymal stem cells
MSCs are a type of adult stem cell that remain in the body during adulthood and continuously renew and differentiate into specialized cells. MSCs are found in various adult tissues, the main source being bone marrow, but they are also found in adipose tissue, amniotic fluid, umbilical cord blood and the placenta11 .
A simple and commonly used method to obtain MSCs is to isolate mononuclear cells from bone marrow and discard all cells that do not adhere to a tissue culture plastic. However, there are limitations to this method, especially regarding specificity, and more advanced methods exist whereby the adherent fraction is purified further based on the presence of one or more MSC markers.
Unlike ESCs (which are pluripotent), MSCs are multipotent, meaning they can only differentiate into a small number of cell types – most notably bone, cartilage, muscle and fat cells. Another key difference is that MSCs cultured in vitro do not divide indefinitely, but instead show a decreasing rate of proliferation over time. For these reasons, some scientists prefer to use the term "multipotent (mesenchymal) stromal cell"12 .
In the human body, MSCs fulfil a variety of roles, for example bone repair after a fracture. When a fracture occurs, MSCs migrate from the bone marrow to the fracture site and differentiate into bone-forming cells that repair the damage13 .
In research, MSCs are studied for treatment of various conditions, including orthopedic injuries, graft-versus-host disease following bone marrow transplantation, cardiovascular, autoimmune and liver diseases. Because MSCs occur naturally in adults, the risk of tumor formation after implantation is low13 .
Lire moins
Applications
The range of research and clinical applications of stem cells is vast. When it comes to in vitro research, common applications include, developmental biology – leading to an enormous increase in our understanding of cell differentiation and the way that tissues, organs and organisms develop – as well as disease modeling and drug discovery.
Stem cells have also captured the publics imagination and generated media coverage across the world, for example when scientists created the first beating heart cells in a petri dish14 . So far, two Nobel prizes in medicine have been awarded for key discoveries in stem cell research. In 2007, Sir Martin Evans, who cultured mouse ESCs in vitro for the first time, received the Nobel prize; in 2012, Shinya Yamanaka received the prize for the discovery of iPSCs.
Despite early setbacks, there has been an increase in clinical trials in recent years with a fastincreasing rate of approvals of iPSC trials in particular, for treatment of high profile conditions, such as Parkinson’s disease, age-related macular degeneration and heart disease15 . If these ongoing trials can demonstrate both safety and efficacy, the number of approvals is expected to rise significantly in the future.
One important aspect in assessing the safety of stem cell treatments is the range of cells and compounds with which the stem cells come in contact during in vitro culture. in particular show a large amount of variation – from animal-derived 'feeder cells' to synthetic, chemically defined surfaces – which can have an impact on cell behavior as well as clinical safety.
The range of research and clinical applications of stem cells is vast. When it comes to in vitro research, common applications include, developmental biology – leading to an enormous increase in our understanding of cell differentiation and the way that tissues, organs and organisms develop – as well as disease modeling and drug discovery.
Stem cells have also captured the publics imagination and generated media coverage across the world, for example when scientists created the first beating heart cells in a petri dish14 . So far, two Nobel prizes in medicine have been awarded for key discoveries in stem cell research. In 2007, Sir Martin Evans, who cultured mouse ESCs in vitro for the first time, received the Nobel prize; in 2012, Shinya Yamanaka received the prize for the discovery of iPSCs.
Despite early setbacks, there has been an increase in clinical trials in recent years with a fastincreasing rate of approvals of iPSC trials in particular, for treatment of high profile conditions, such as Parkinson’s disease, age-related macular degeneration and heart disease15 . If these ongoing trials can demonstrate both safety and efficacy, the number of approvals is expected to rise significantly in the future.
One important aspect in assessing the safety of stem cell treatments is the range of cells and compounds with which the stem cells come in contact during in vitro culture. in particular show a large amount of variation – from animal-derived 'feeder cells' to synthetic, chemically defined surfaces – which can have an impact on cell behavior as well as clinical safety.
Lire moins
References
1.
2. Thomson JA et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998;282(5391): 1145–1147.
3.
4. Cyranoski D. How human embryonic stem cells sparked a revolution. Nature 2018.
5. Takahashi K and Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006;126(4): 663–76.
6. Yoshida Y et al. Hypoxia Enhances the Generation of Induced Pluripotent Stem Cells. Cell Stem Cell 2009;5(3): 237–241.
7. Kim JB et al. Direct reprogramming of human neural stem cells by OCT4. Nature 2009;461: 649–654.
8. Soldner F et al. Parkinson's Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009;136(5): 964–977.
9. Deng XY et al. Non-Viral Methods For Generating Integration-Free, Induced Pluripotent Stem Cells. Current Stem Cell Research and Therapies 2015;10(2): 153–158.
10. Scudellari M. How iPS cells changed the world. Nature 2016;534: 310–312.
11. Galipeau J et al. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018;22: 824–833.
12. Dominici M et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4): 315–317.
13. Kim N et al. Clinical applications of mesenchymal stem cells. Korean Journal for Internal Medicine 2013;28(4): 387–402.
14.
15. Cyranoski D. 'Reprogrammed' stem cells to be tested in people with Parkinson's. Nature 2018.
2. Thomson JA et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998;282(5391): 1145–1147.
3.
4. Cyranoski D. How human embryonic stem cells sparked a revolution. Nature 2018.
5. Takahashi K and Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006;126(4): 663–76.
6. Yoshida Y et al. Hypoxia Enhances the Generation of Induced Pluripotent Stem Cells. Cell Stem Cell 2009;5(3): 237–241.
7. Kim JB et al. Direct reprogramming of human neural stem cells by OCT4. Nature 2009;461: 649–654.
8. Soldner F et al. Parkinson's Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009;136(5): 964–977.
9. Deng XY et al. Non-Viral Methods For Generating Integration-Free, Induced Pluripotent Stem Cells. Current Stem Cell Research and Therapies 2015;10(2): 153–158.
10. Scudellari M. How iPS cells changed the world. Nature 2016;534: 310–312.
11. Galipeau J et al. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018;22: 824–833.
12. Dominici M et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4): 315–317.
13. Kim N et al. Clinical applications of mesenchymal stem cells. Korean Journal for Internal Medicine 2013;28(4): 387–402.
14.
www.bhf.org.uk/informationsupport/heart-mattersmagazine/research/breakthroughs-in-stem-cell-research
15. Cyranoski D. 'Reprogrammed' stem cells to be tested in people with Parkinson's. Nature 2018.
Lire moins
